Manganese mining area wastewater treatment method
A technology for wastewater treatment and mining areas, applied in mining wastewater treatment, water/sewage treatment, multi-stage water/sewage treatment, etc., to achieve the effects of easy implementation, low reagent cost, and improved concrete strength
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
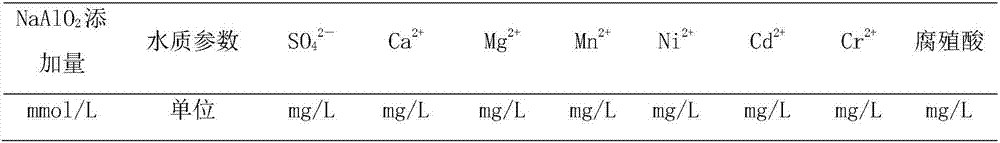
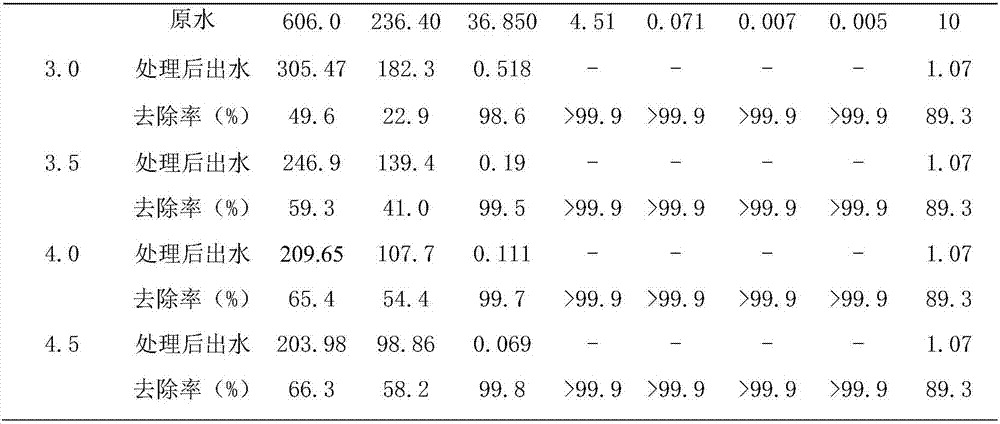
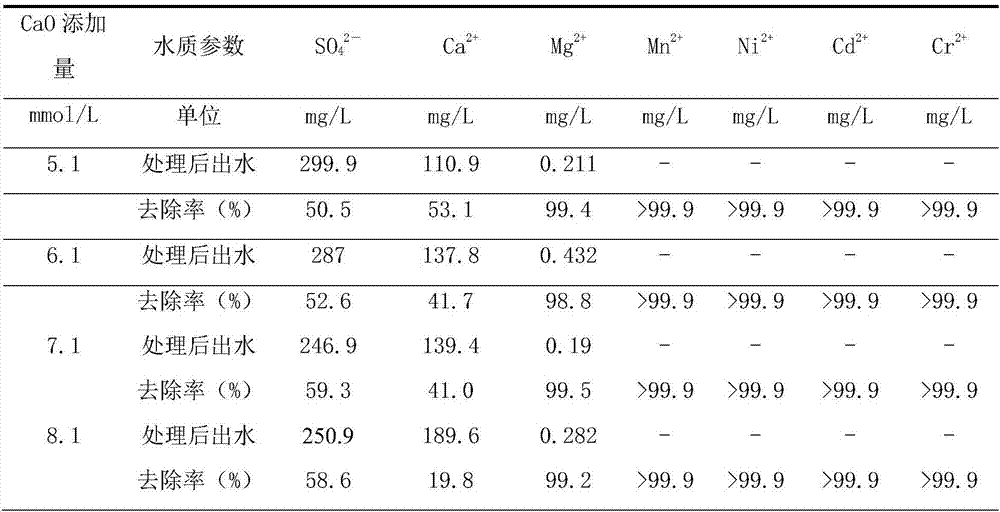
[0042] This embodiment selects the polluted water body of a manganese mining area in Guangxi, the sulfate radical concentration in the water is 606.0mg / L, the calcium ion concentration is 236.4mg / L, the magnesium ion concentration is 36.85mg / L, and the manganese ion concentration is 4.51mg / L, pH is 6.99, other water quality parameters are as shown in table 1, add the humic acid of 10mg / L in described water sample, to test the effect that the present invention removes organic matter. The treatment temperature for removing sulfate radicals and other metal ions and humic acid by the process of the present invention is 20°C.
[0043] S1. Add 3.5mmol / L NaAlO 2 , 7.1mmol / L CaO, so that the Ca in the water sample 2+ / Al 3+ / SO 4 2- The initial molar ratio is 2.06:0.56:1, and the pH of the water sample is 12.14;
[0044] S2. Stir at a speed of 300r / min for 60min, and remove the precipitate after standing for 30min;
[0045] S3. Adjust the pH of the water sample to 6.5, and stir ...
Embodiment 2
[0052] In this embodiment, a polluted water body in a manganese mining area in Guangxi is selected, and the water quality parameters are shown in Table 1. The treatment temperature was 20°C.
[0053] Repeat Example 1 with the same steps described, the difference is that in the step S1, add 3.0mmol / LNaAlO 2 , 7.1mmol / L CaO, so that the Ca in the water sample 2+ / Al 3+ / SO 4 2- The initial molar ratio was 2.06:0.48:1 and the pH was 12.14. The concentrations of ions in the effluent after treatment are shown in Table 1.
Embodiment 3
[0055] In this embodiment, a polluted water body in a manganese mining area in Guangxi is selected, and the water quality parameters are shown in Table 1. The treatment temperature was 20°C.
[0056] Repeat Example 1 with the same steps described, the difference is that in the step S1, add 4.0mmol / LNaAlO 2 , 7.1mmol / L CaO, so that the Ca in the water sample 2+ / Al 3+ / SO 4 2- The initial molar ratio was 2.06:0.63:1 and the pH was 12.14. The concentrations of ions in the effluent after treatment are shown in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com