Ligustrazine acylhydrazone derivatives, and preparation method and application thereof
A technology of ligustrazine acyl hydrazone and ligustrazine hydrazide is applied in the field of ligustrazine acyl hydrazone derivatives, which can solve the problems of high normal cytotoxicity, low yield, low antitumor activity and the like, and achieves low normal cytotoxicity, Simple synthetic route and strong antitumor activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
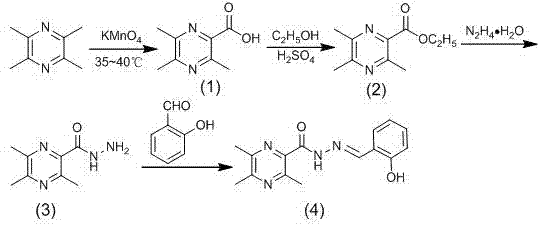
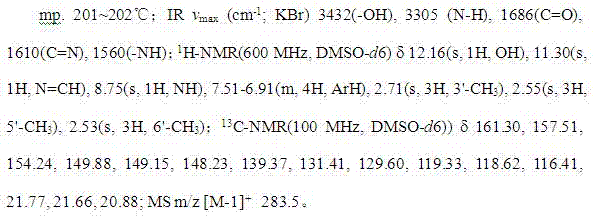
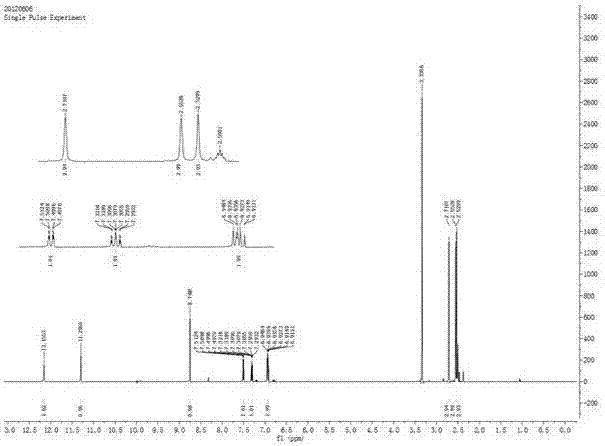
[0045] Example 1 A preparation method of ligustrazine acylhydrazone derivatives
[0046] Include the following steps:
[0047] (1) Preparation of Ligustrazinic Acid
[0048] 8.4% KMnO 4 Aqueous solution (contains KMnO 4 6.32g) was added dropwise to 3.8g ligustrazine, and the dropwise addition was completed within 60 minutes. The reaction temperature was controlled at 35°C, reacted for 24 hours, filtered, the filtrate was concentrated under reduced pressure, cooled in an ice-water bath, adjusted to pH 1 with dilute hydrochloric acid, and kept in the refrigerator overnight Refrigerate, extract with chloroform, concentrate under reduced pressure, and then recrystallize with 95% ethanol to obtain ligustrazinic acid as a light yellow crystalline powder.
[0049] (2) Preparation of Ethyl Ligustrazinate
[0050] Add 0.75mL (0.0138mol) of concentrated sulfuric acid to 1.66g (0.01mol) of ligustracinic acid and 17mL of absolute ethanol (0.29mol), and reflux for 24h. Concentrate und...
Embodiment 2
[0056] Embodiment 2 A kind of preparation method of ligustrazine acylhydrazone derivatives
[0057] Include the following steps:
[0058] (1) Preparation of Ligustrazinic Acid
[0059] Will contain 8.4% KMnO 4 Aqueous solution (contains KMnO 4 63.2g) was added dropwise to 38g ligustrazine, and the dropwise addition was completed within 60 minutes. The reaction temperature was controlled at 35°C, reacted for 24 hours, filtered, the filtrate was concentrated under reduced pressure, cooled in an ice-water bath, adjusted to pH 1 with dilute hydrochloric acid, and refrigerated overnight , extracted with chloroform, concentrated under reduced pressure, and then recrystallized with 95% ethanol to obtain ligustrazinic acid as a pale yellow crystalline powder.
[0060] (2) Preparation of Ethyl Ligustrazinate
[0061] Add 1.5mL (0.0276mol) of concentrated sulfuric acid to 16.6g (0.1mol) of ligustracinic acid and 170mL (2.9mol) of absolute ethanol, and reflux for 16h. Concentrate un...
Embodiment 3
[0067] Embodiment 3 A kind of preparation method of ligustrazine acylhydrazone derivatives
[0068] Include the following steps:
[0069] (1) Preparation of Ligustrazinic Acid
[0070] Will contain 8.4% KMnO 4 Aqueous solution (contains KMnO 4 3.16g) was added dropwise to 19g ligustrazine, and the dropwise addition was completed within 60 minutes. The reaction temperature was controlled at 35°C, reacted for 24 hours, filtered, the filtrate was concentrated under reduced pressure, cooled in an ice-water bath, adjusted to pH 1 with dilute hydrochloric acid, and refrigerated overnight , extracted with chloroform, concentrated under reduced pressure, and then recrystallized with 95% ethanol to obtain ligustrazinic acid as a pale yellow crystalline powder.
[0071] (2) Preparation of Ethyl Ligustrazinate
[0072] Add 0.86mL (0.0158mol) of concentrated sulfuric acid to 8.2g (0.049mol) of ligustracinic acid and 83mL of absolute ethanol (1.42mol), and reflux for 16h. Concentrate ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com