Method for preparing polylactone through ring opening
一种聚内酯、开环聚合的技术,应用在有机催化和高分子材料领域,能够解决没有完全代替DMAP使用等问题,达到降低不利影响、应用广泛、条件简单的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
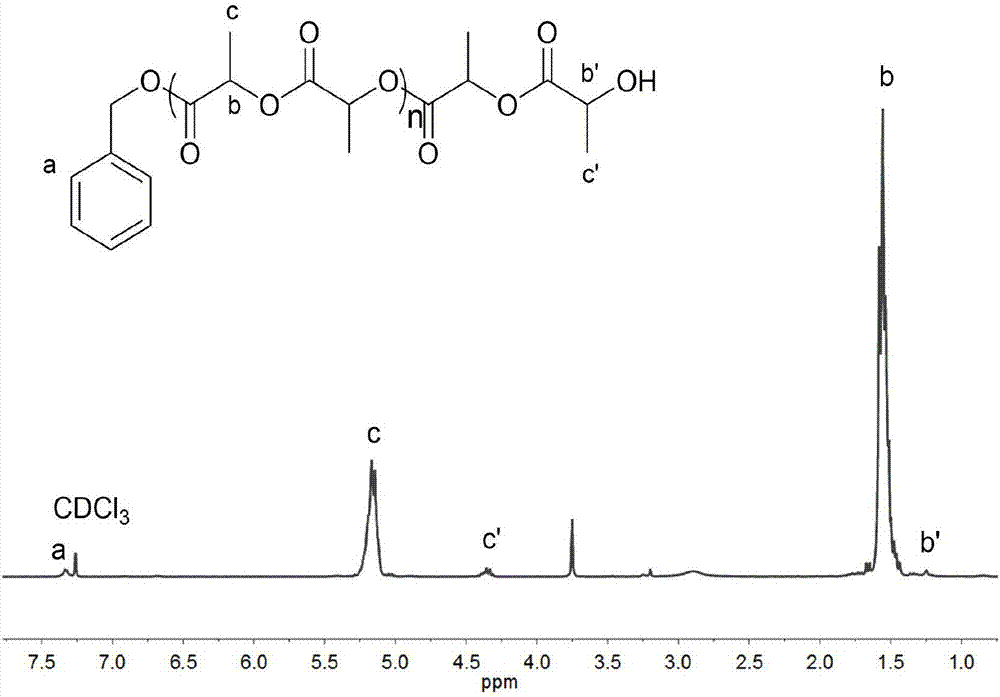
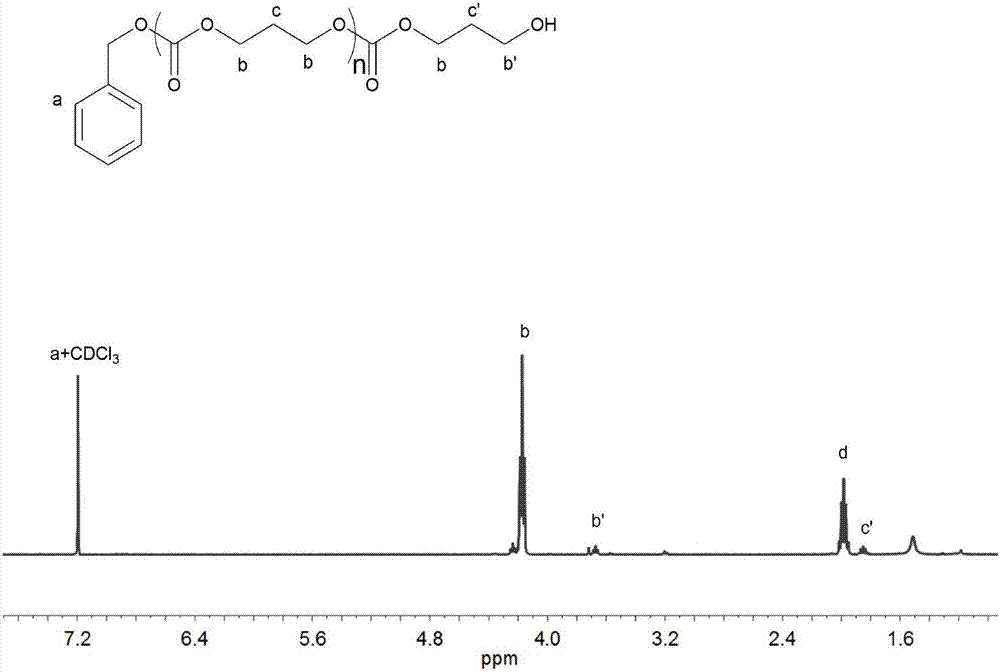
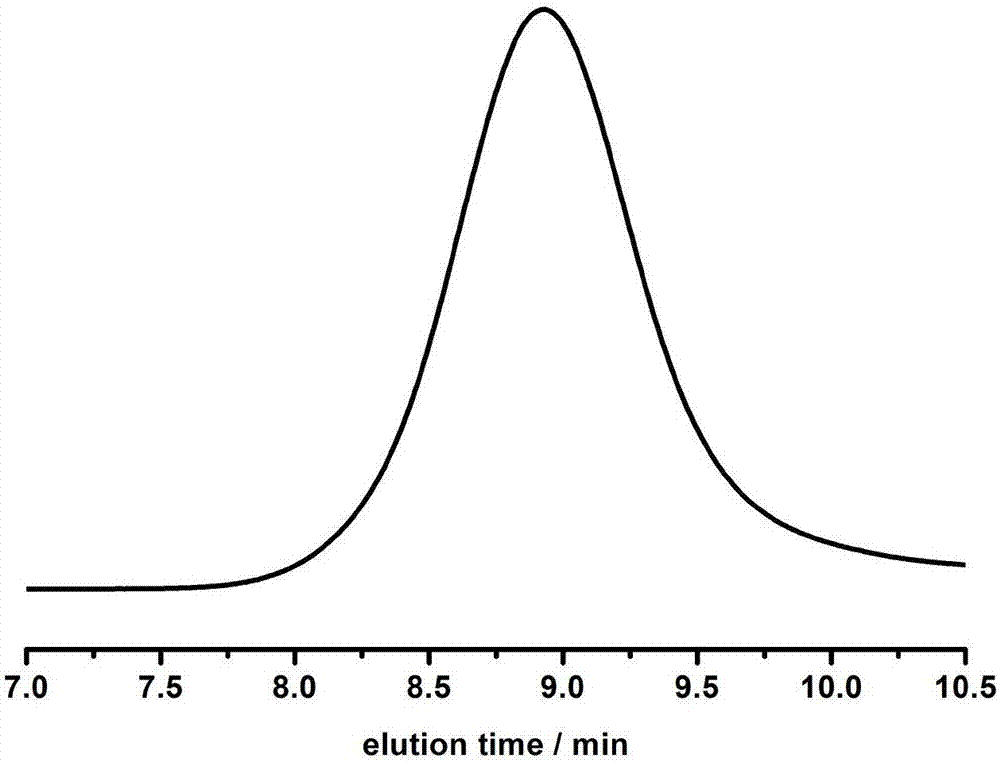
[0047] In a 3ml polymerization tube, add L-lactide (0.4147g, 2.88mmol, 30equiv), catalyst (compound as No. 16) (0.0152g, 0.096mmol, 1.0equiv), benzyl alcohol (10μL, 0.096mmol , 1.0equiv), under the condition of 140 ℃, mechanically stirred for 1.5 hours. After the reaction was over, the reaction was terminated. The obtained crude product was slowly dropped into cold methanol, and the polymer was obtained by centrifugal precipitation, which was filtered and dried to constant weight to obtain 0.3855g white Solid, conversion rate 96.0%, number average molecular weight M n It is 4300g / mol, and the degree of dispersion PDI is 1.03.
Embodiment 2
[0049] In a 3ml polymerization tube, add L-lactide (1.660g, 11.52mmol, 120equiv), catalyst (compound as shown in No. 16) (0.0152g, 0.096mmol, 1.0equiv), benzyl alcohol (10μL, 0.096mmol , 1.0equiv), mechanically stirred for 7 hours at 140°C. After the reaction was over, the reaction was terminated. The obtained crude product was slowly dropped into cold methanol, and the polymer was obtained by centrifugal precipitation, which was filtered and dried to constant weight to obtain 1.403g white Solid, conversion rate 84.7%, number average molecular weight M n It is 13610g / mol, and the dispersion PDI is 1.04.
Embodiment 3
[0051] In a 3ml polymerization tube, add trimethylene carbonate (0.2937g, 2.88mmol, 30equiv), catalyst (compound as shown in No. 16) (0.0152g, 0.096mmol, 1.0equiv), benzyl alcohol (10μL, 0.096mmol , 1.0equiv), mechanically stirred at 60°C for 24 hours. After the reaction was over, the reaction was terminated. The obtained crude product was slowly dropped into cold methanol, and the polymer was obtained by centrifugal precipitation, filtered and dried to constant weight to obtain 0.2620g transparent Oil, conversion rate 91.0%, number average molecular weight M n It is 2850g / mol, and the degree of dispersion PDI is 1.04.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight dispersion degree | aaaaa | aaaaa |
| molecular weight dispersion degree | aaaaa | aaaaa |
| molecular weight dispersion degree | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com