Styrylphenol polyoxyethylene ether and preparation method thereof
A technology of styryl phenol and polyoxyethylene ether, which is applied in the field of styryl phenol polyoxyethylene ether and its preparation, can solve the problems of wide distribution, deep color and the like, and achieves narrow distribution, light color and strong selectivity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
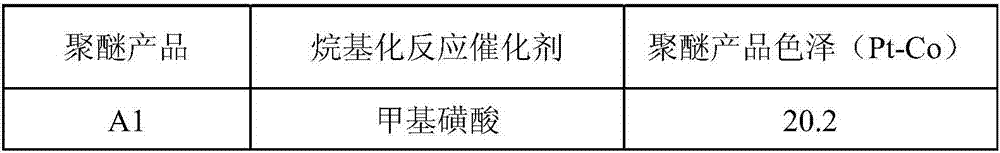
[0031] Embodiment 1: the influence of phenol alkylation reaction catalyst on product color and luster
[0032] Alkylation reaction: Weigh 253.4g of phenol and 2.03g of methanesulfonic acid catalyst into a four-neck flask, put nitrogen and raise the temperature to 90°C, add 560.6g of styrene dropwise under rapid stirring, complete the dropwise addition in 1.5 hours, and then keep the temperature until The refractive index (25°C) of the product reaches 1.5985-1.6020, the reaction is completely cooled to room temperature, and NaOH is added to adjust the pH to 7.0 to obtain 814g of styrylphenol.
[0033] Ethylene oxide polyaddition reaction: Weigh 500g of styrylphenol into the autoclave, heat up to 100°C under nitrogen, dehydrate in vacuum for 1 hour, then cool down to 40°C, add 0.55g of organic phosphazene salt, heat up to 100°C under nitrogen , 728.5g of ethylene oxide was added, the reaction was complete at 95°C and 0.025MPa, and 1228.5g of styrylphenol polyoxyethylene ether (a...
Embodiment 2
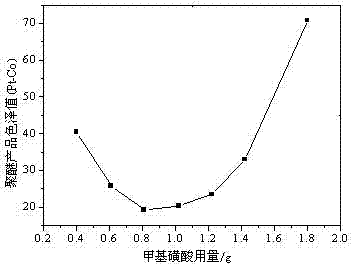
[0040] Embodiment 2: the influence of methylsulfonic acid catalyst dosage on product color and luster
[0041] On the basis of embodiment 1, continue to test the optimal phenol alkylation reaction catalyst selected, i.e. the addition of methanesulfonic acid, specifically expressed as follows:
[0042] (1) Alkylation reaction: Weigh 126.7g of phenol and 1.02g of methanesulfonic acid catalyst into a four-neck flask, put nitrogen and raise the temperature to 90°C, add 280.3g of styrene dropwise under rapid stirring, and dropwise add in 1.5 hours, then Insulate the reaction until the product's refractive index (25°C) reaches 1.5985-1.6020, and the reaction is completely cooled to room temperature. NaOH is added to adjust the pH to 7.0, and 407g of styrylphenol is obtained.
[0043] (2) Ethylene oxide polyaddition reaction: add styryl phenol into the autoclave, heat up to 100°C under nitrogen, cool down to 40°C after vacuum dehydration for 1 hour, add 0.45 g of organic phosphazene ...
Embodiment 3
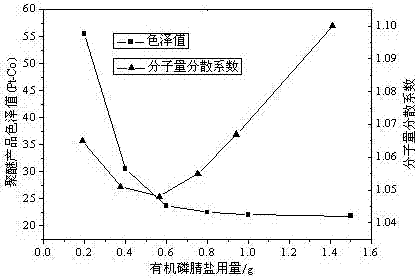
[0046] Embodiment 3: the impact of ethylene oxide polyaddition catalyst on product index
[0047] Alkylation reaction: Weigh 126.7g of phenol and 0.81g of methanesulfonic acid catalyst into a four-necked flask, put nitrogen and raise the temperature to 90°C, add 280.3g of styrene dropwise under rapid stirring, complete the dropwise addition in 1.5 hours, and then keep warm for the reaction until The refractive index (25°C) of the product reaches 1.5985-1.6020, the reaction is completely cooled to room temperature, and NaOH is added to adjust the pH to 7.0 to obtain 407g of styrylphenol.
[0048] Ethylene oxide polyaddition reaction: Weigh 407g of styrylphenol into the autoclave, heat up to 100°C under nitrogen, dehydrate in vacuum for 1 hour, then cool down to 40°C, add 0.6g of organic phosphazene salt, heat up to 100°C under nitrogen , add 593g of ethylene oxide, complete the reaction at 100°C and 0.035MPa, and cool down to room temperature to obtain styrene-based phenol poly...
PUM
| Property | Measurement | Unit |
|---|---|---|
| refractive index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com