Method for separating and preparing natural naphthoquinone compounds
A compound and separation method technology, applied in the field of medicine, can solve the problems of difficulty in simultaneous separation and time-consuming, and achieve the effects of simple operation and high compound purity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
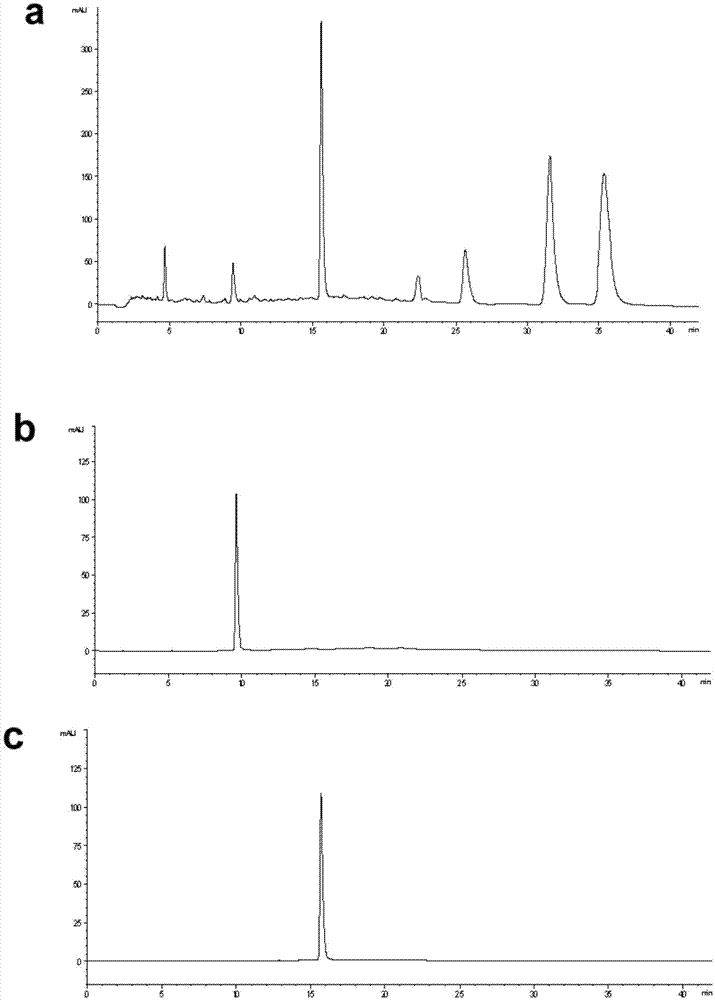
Embodiment 1
[0042] (1) In this embodiment, the OptiChrome-300PLUS high-speed countercurrent chromatograph produced by Jiangyin Countercurrent Technology Co., Ltd. is improved, and the Agilent 1200HPLC high-performance liquid chromatograph produced by Agilent Co., Ltd. of the United States is used for purity analysis;
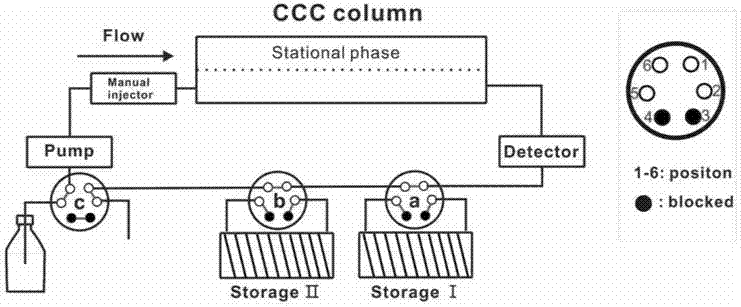
[0043] Set up a high-speed countercurrent chromatograph, in which, after the detector of the high-speed countercurrent chromatograph, flow valves a and b are connected, six-way valves a and b are respectively connected to storage rings I and II, and six-way valve c is connected in front of the constant flow pump, which is responsible for switching the normal washing Detachment mode and circulation elution mode, (the structure diagram of the high-speed countercurrent chromatograph of setting is as follows figure 1 shown);
[0044] (2) Preparation of extract samples
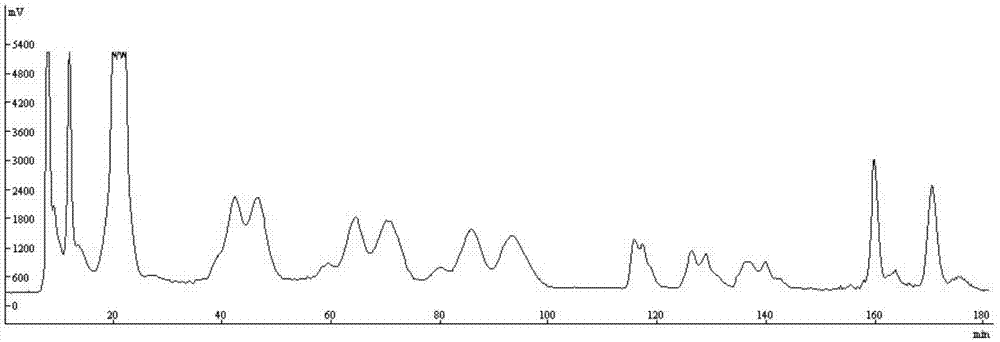
[0045] 54.8g of dried whole herb of Xinjiang comfrey was ultrasonically extracted three times with 500mL of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com