Vaccine Lyoprotectant
A freeze-dried protective agent and vaccine technology, applied in vaccines, veterinary vaccines, inorganic non-effective ingredients, etc., can solve the problem of poor protection function, poor heat resistance of freeze-dried live vaccines, limited storage and transportation of freeze-dried live vaccines, etc. problem, to achieve the effect of improving heat resistance, excellent protection performance, and prolonging storage time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
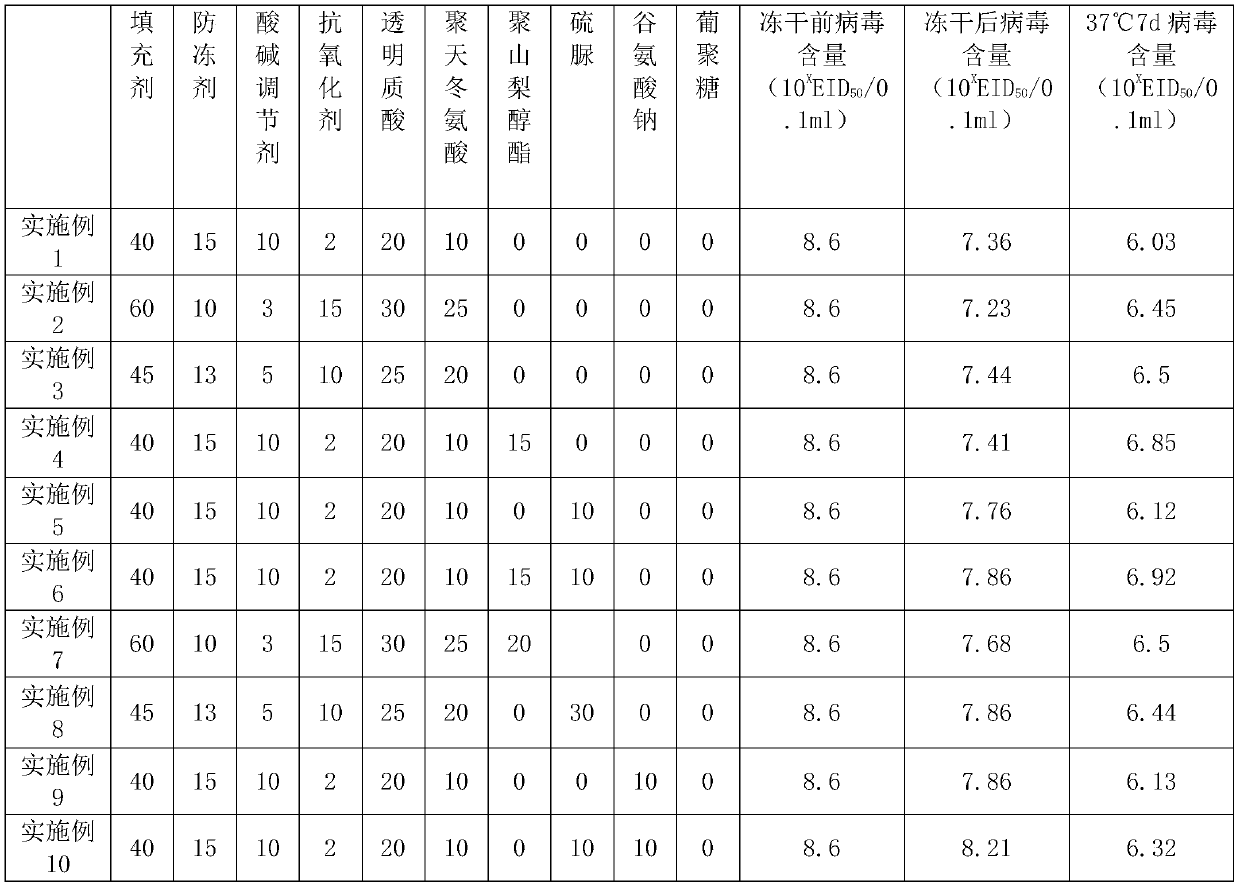
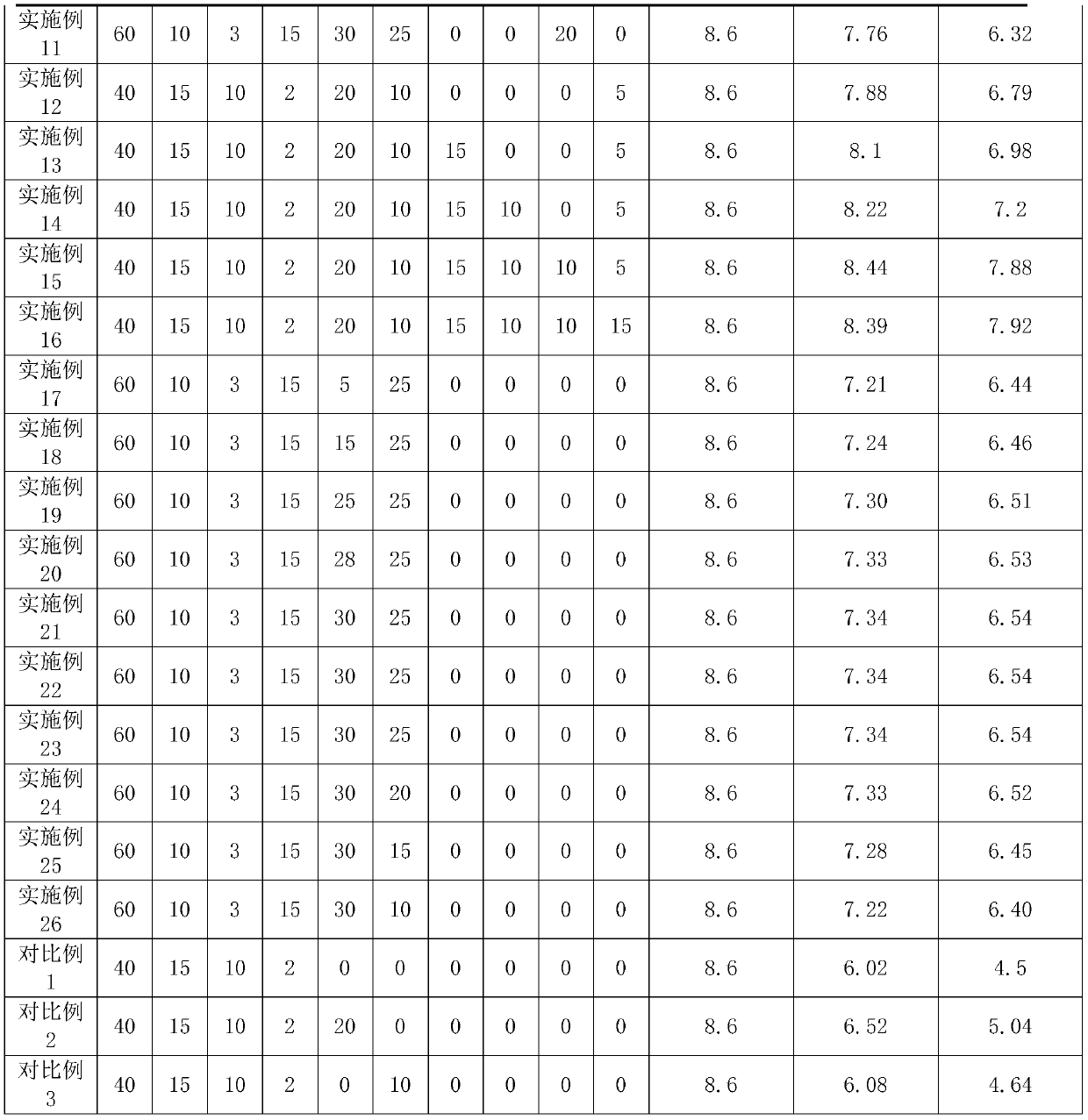
[0030] Example 1: Vaccine lyoprotectant, in parts by weight, weighed the components in the following Table 1, prepared solutions with ultrapure water, mixed evenly after sterilization, and obtained vaccine lyoprotectant . Among them, the filler is gelatin, the antifreeze is glycerin, the antioxidant is vitamin D, the acid-base regulator is phosphate buffer, and the phosphate buffer is configured as follows: 0.2mol / L sodium dihydrogen phosphate solution and 0.2mol / L 1 L of disodium hydrogen phosphate aqueous solution, weigh 36ml of the above-mentioned sodium dihydrogen phosphate and 14ml of the above-mentioned disodium hydrogen phosphate, and mix them to obtain a phosphate buffer.
[0031] Preparation of freeze-dried live vaccine: Commercially purchased chicken Xincheng La Sota strain and chicken embryos.
[0032] Determination of virus content before freeze-drying: According to the requirements of "Chinese Pharmacopoeia" three (2010): make 10-fold serial dilutions of the toxi...
Embodiment 2
[0036] Embodiment 2: Vaccine lyoprotectant, the difference from Embodiment 1 is that the filler is sucrose, the antifreeze is dimethyl sulfoxide, and the antioxidant is vitamin E. Table 1 lists the virus content before freeze-drying, the virus content after freeze-drying, and the results of the thermal stability test of the freeze-dried vaccine.
Embodiment 3
[0037] Embodiment 3: Vaccine lyoprotectant, the difference from Embodiment 1 is that the filler is mannitol, and the antioxidant is sodium thiosulfate. Table 1 lists the virus content before freeze-drying, the virus content after freeze-drying, and the results of the thermal stability test of the freeze-dried vaccine.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com