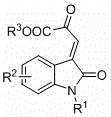
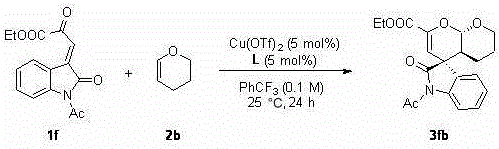
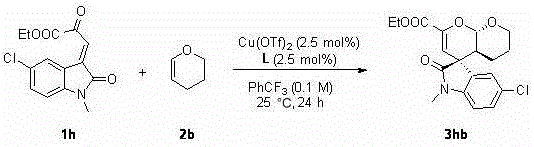
Chiral spiro oxindole dihydropyran derivative and synthesizing method thereof
A technology of spirocyclic oxindole dihydropyran derivatives and synthetic methods, applied in the field of chiral spirocyclic oxindole dihydropyran derivatives and their catalytic synthesis, achieving high yield and chemoselectivity Good, handles simple effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031]
[0032] Under nitrogen atmosphere, Cu(OTf) 2 (0.9 mg, 0.0025 mmol, 2.5 mol%), L (1.84 mg, 0.0025 mmol, 2.5 mol%) were placed in anhydrous benzotrifluoride (1 mL), stirred at room temperature for 1 hour, and reactant 1a (25.9 mg, 0.1 mmol) and 2b (16.8 mg, 0.2 mmol, 2 equiv), reacted at room temperature for 24 hours until the substrate 1a disappeared, and the reaction system was directly separated by petroleum ether / ethyl acetate (3 / 1) column chromatography to obtain 32.2 mg White solid 3ab, white solid, 94% yield, 161–162 °C.
[0033] The product 3ab was analyzed and the results were as follows: >99:1 dr , 94% ee [Daicel Chiralcel AD-H, hexanes / i -PrOH = 80 / 20, flow rate: 1.0 mL·min –1 , λ = 254.4 nm, t (major)=12.087, t (minor)= 15.133]; [ α ]25 D = 148.9 (c 0.14, CHCl 3 ); 1 H NMR (400 MHz, CDCl 3 ) δ 7.31 (td, J = 7.6, 1.7 Hz, 1H), 7.16 – 7.02 (m, 2H), 6.84 (d, J =7.8 Hz, 1H), 5.67 (s, 1H), 5.60 (d, J = 8.9 Hz, 1H), 4.23 (qq, J = 7.5, 3.7Hz, 2...
Embodiment 2
[0036]
[0037] Under nitrogen atmosphere, Cu(OTf) 2 (1.8 mg, 0.005 mmol, 5 mol%), L (3.69 mg, 0.005 mmol, 5 mol%) were placed in anhydrous benzotrifluoride (1 mL), stirred at room temperature for 1 hour, and reactant 1b (24.5 mg, 0.1mmol) and 2b (33.6 mg, 0.4 mmol, 4 equiv), reacted at room temperature for 24 hours until the substrate 1b disappeared, and the reaction system was directly separated by petroleum ether / ethyl acetate (3 / 1) column chromatography to obtain 29.9 mg White solid 3bb, white solid, 91% yield, 140–141 °C.
[0038] Product 3bb is analyzed, and the results are as follows: 96:4 dr , 93% ee [Daicel Chiralcel AD-H, hexanes / i -PrOH = 80 / 20, flow rate: 1.0mL min –1 , λ = 254.4 nm, t (major)=13.307, t (minor)= 18.928]; [ α ]25 D = 191.7 (c 0.1, CHCl 3 ); 1 H NMR (400 MHz, CDCl 3 ) δ 7.33 (td, J = 7.5, 1.8 Hz, 1H), 7.16 – 7.06 (m, 2H), 6.85 (d, J =7.8 Hz, 1H), 5.70 (s, 1H), 5.63 (d, J = 8.9 Hz, 1H), 4.09 (dd, J = 11.8, 4.6Hz, 1H), 3.80 (s, 3...
Embodiment 3
[0041]
[0042] Under nitrogen atmosphere, Cu(OTf) 2 (0.9 mg, 0.0025 mmol, 2.5 mol%), L (1.84 mg, 0.0025 mmol, 2.5 mol%) were placed in anhydrous benzotrifluoride (1 mL), stirred at room temperature for 1 hour, and reactant 1c (28.9 mg, 0.1 mmol) and 2b (16.8 mg, 0.2 mmol, 2 equiv), reacted at room temperature for 24 hours until the substrate 1c disappeared, and the reaction system was directly separated by petroleum ether / ethyl acetate (3 / 1) column chromatography to obtain 34.6 mg White solid 3cb, white solid, 93% yield, 63–64 °C.
[0043] The product 3cb was analyzed and the results were as follows: >99:1 dr , 93% ee [Daicel Chiralcel AD-H, hexanes / i -PrOH = 80 / 20, flow rate: 1.0 mL·min –1 , λ = 254.4 nm, t (major)=10.943, t (minor)= 12.549]; [ α ]25 D = 185 (c 0.16, CHCl 3 ); 1 H NMR (400 MHz, CDCl 3 ) δ 7.31 (s, 1H), 7.18 – 7.08 (m, 2H), 7.04 (d, J = 7.9 Hz, 1H), 5.69(d, J = 1.6 Hz, 1H), 5.59 (dd, J = 9.0, 1.6 Hz, 1H), 5.21 – 4.99 (m, 2H),4.25 (qq, J ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com