Tetrabutylammonium hydrogen sulfate buffer salt system for liquid chromatogram detection
A technology of tetrabutylammonium bisulfate and buffer salt, which is applied in the direction of measuring devices, instruments, scientific instruments, etc., can solve the problems of low utilization rate of instruments at night, inability to analyze continuously at night, and inability to prepare too much buffer salt, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Preparation and stability observation of embodiment 1 mobile phase
[0020] 1. Reagent source:
[0021] Water, water for chromatography, self-made;
[0022] Tetrabutylammonium bisulfate, analytically pure, Shanghai Junrui Biotechnology Co., Ltd.;
[0023] Potassium dihydrogen phosphate, analytically pure, Shanghai Junrui Biotechnology Co., Ltd.;
[0024] Phosphoric acid, content 85%, analytically pure, Nanjing Saihongrui Biotechnology Co., Ltd.;
[0025] Dipotassium EDTA, analytically pure, Beijing Baierdi Biotechnology Co., Ltd.
[0026] 2. Preparation method
[0027] Buffer system of the present invention: Weigh 0.5 g of tetrabutylammonium hydrogen sulfate, 1 g of potassium dihydrogen phosphate, 2 mL of phosphoric acid with a content of 85%, 50 mg of dipotassium ethylenediamine tetraacetate, dissolve them in 500 mL of water, dilute with water to 1 L .
[0028] Contrast buffer system: According to the conventional method, weigh 0.5 g of tetrabutylammonium hydroge...
Embodiment 2
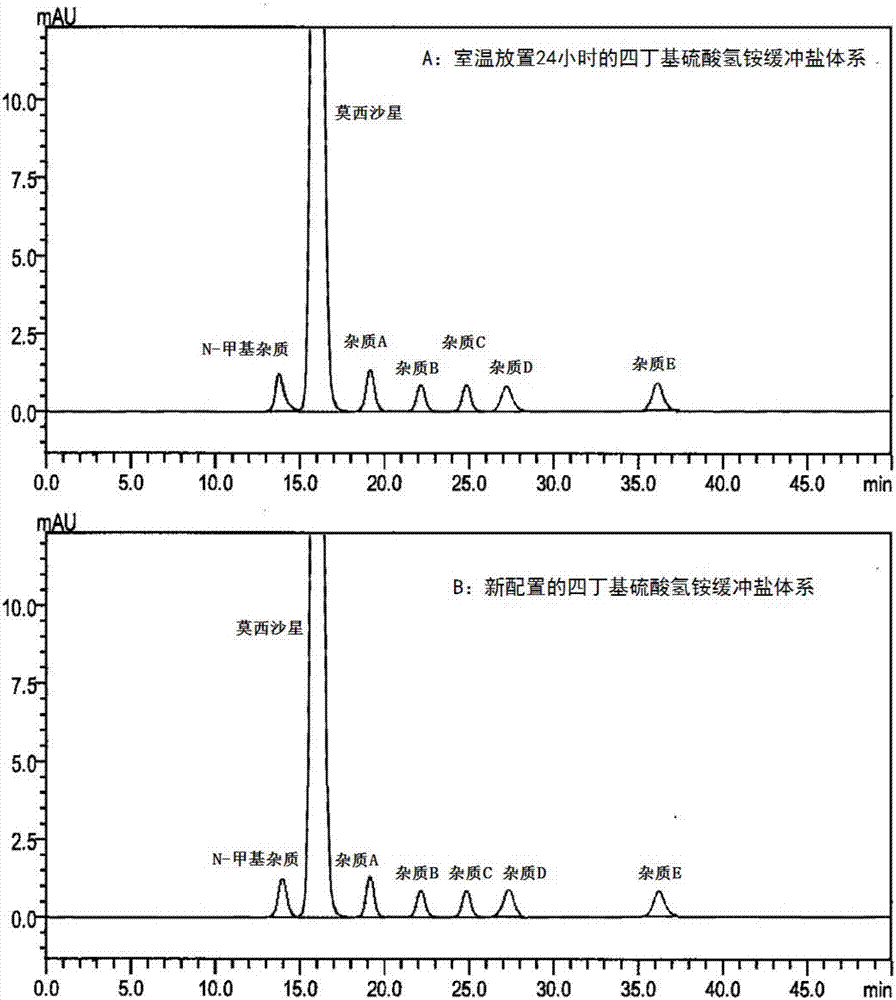
[0031] Example 2 Take Moxifloxacin Hydrochloride Injection (20mL: 400mg) as an example to investigate the effect of tetrabutylammonium bisulfate buffered salt system placed at room temperature for 24 hours and the newly configured tetrabutylammonium bisulfate buffered salt system on moxifloxacin, Differences in the chromatographic behavior of N-methyl impurities, impurity A, impurity B, impurity C, impurity D, and impurity E on reversed-phase chromatographic columns
[0032] 1. Preparation of test solution
[0033] Moxifloxacin hydrochloride injection and N-methyl impurity, impurity A, impurity B, impurity C, impurity D, impurity E standard were prepared according to conventional methods to contain 1 mg of moxifloxacin and N-methyl impurity, impurity A , B, C, D, E each 1μg of the test solution as the test solution.
[0034] 2. HPLC analysis method
[0035] Chromatographic column: Agilent Eclipse Plus Phenyl-Hexyl, 4.6mm×150mm, 5μm;
[0036] Detection wavelength: 293nm;
...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com