Preparation method of octafluorocyclopentene
A technology of octafluorocyclopentene and chloroheptafluorocyclopentene, which is applied in the field of preparation of octafluorocyclopentene by fluorination, can solve the problems of difficult recycling of fluorinated reagent reaction solvents, environmental pollution, etc., and achieve novel routes , reduce pollution and achieve zero pollution effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
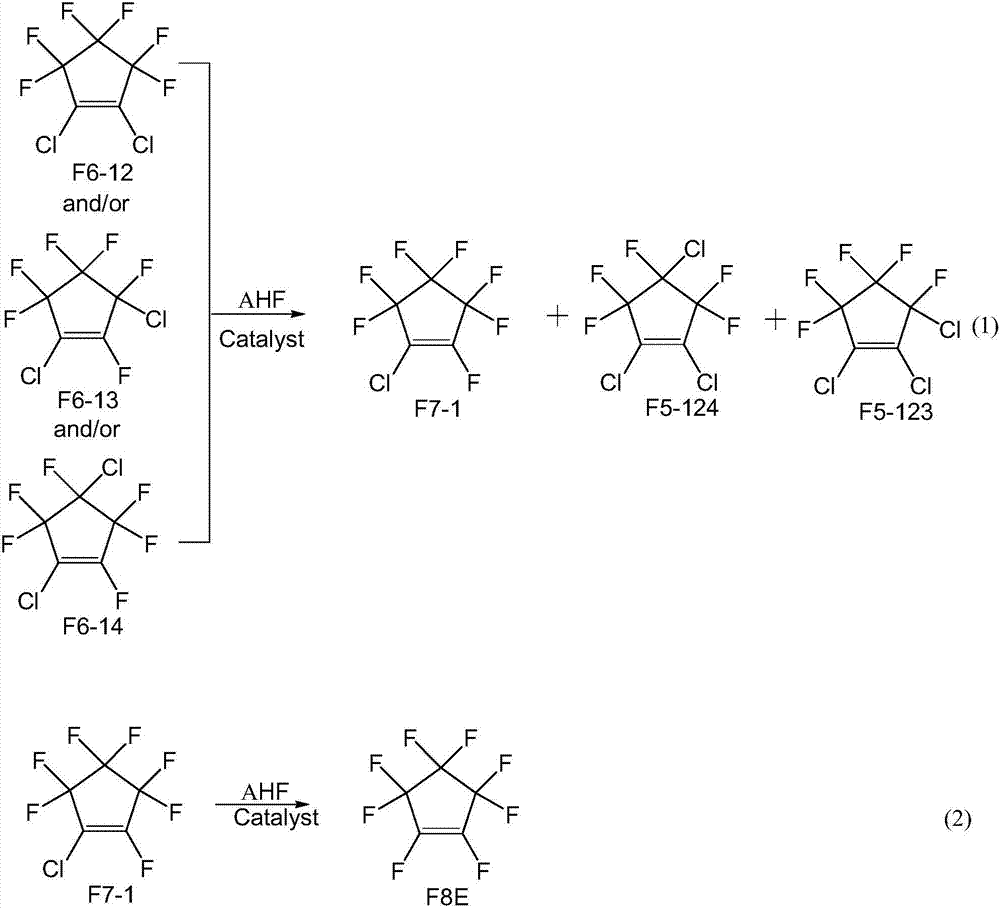
[0035] Preparation of fluorination catalyst: according to the mass percentages of chromium and magnesium as 90% and 10%, dissolve chromium chloride and magnesium chloride in water, add an appropriate amount of precipitant ammonia water at 60°C, and control the pH of the solution to 7.5- 8.5 range, make it fully precipitate under stirring conditions, filter the formed slurry, wash with deionized water until neutral, and then dry at 150°C for 12 hours to obtain the precursor of the catalyst. The precursor of the above catalyst was press-molded, and then calcined at 450°C for 10 hours under a nitrogen atmosphere, and then activated at 300°C for 10 hours with a mixed gas composed of hydrogen fluoride and hydrogen at a molar ratio of 10:1,
[0036] A fluorination catalyst is prepared.
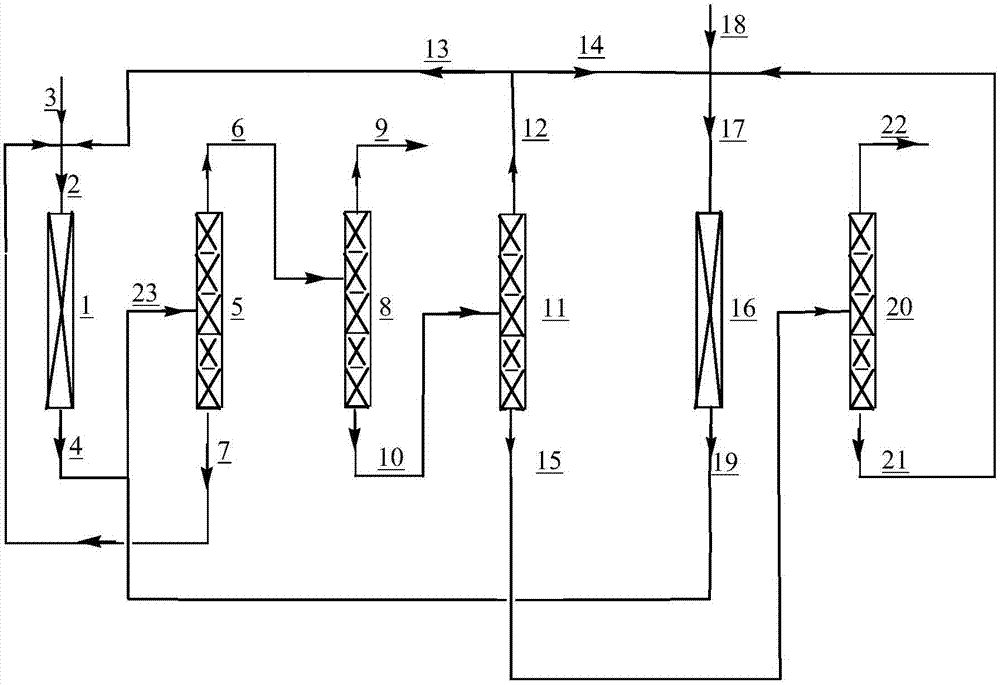
[0037] A tubular reactor made of Inconium alloy with an inner diameter of 1 / 2 inch and a length of 30 cm was filled with 10 ml of the above-prepared fluorination catalyst. The reactor of the first ...
Embodiment 2
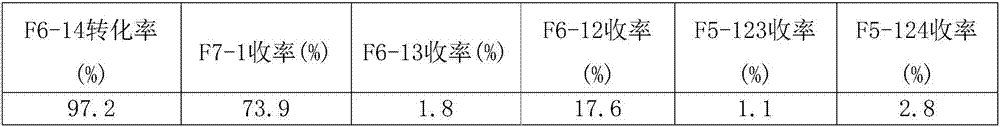
[0042] The same operation as in Example 1, except that 1,2-dichlorohexafluorocyclopentene was changed to an equivalent amount of 1,4-dichlorohexafluorocyclopentene, and the reaction temperature was changed to 290°C, the result See Table 2.
[0043] Table 2
[0044]
[0045] Note: F5-123 is 1,2,3-trichloropentafluorocyclopentene; F5-124 is 1,2,4-trichloropentafluorocyclopentene.
Embodiment 3
[0047] The same operation as in Example 1, except that 1,2-dichlorohexafluorocyclopentene was changed to an equivalent amount of 1,3-dichlorohexafluorocyclopentene, and the reaction temperature was changed to 290°C, the result See Table 3.
[0048] table 3
[0049]
[0050] Note: F5-123 is 1,2,3-trichloropentafluorocyclopentene; F5-124 is 1,2,4-trichloropentafluorocyclopentene.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com