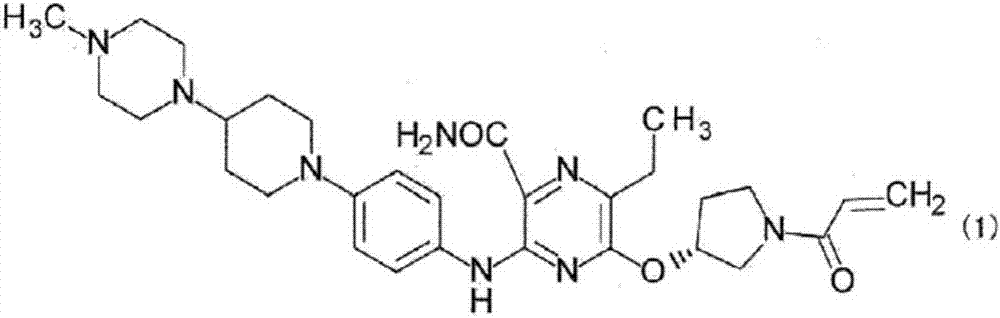
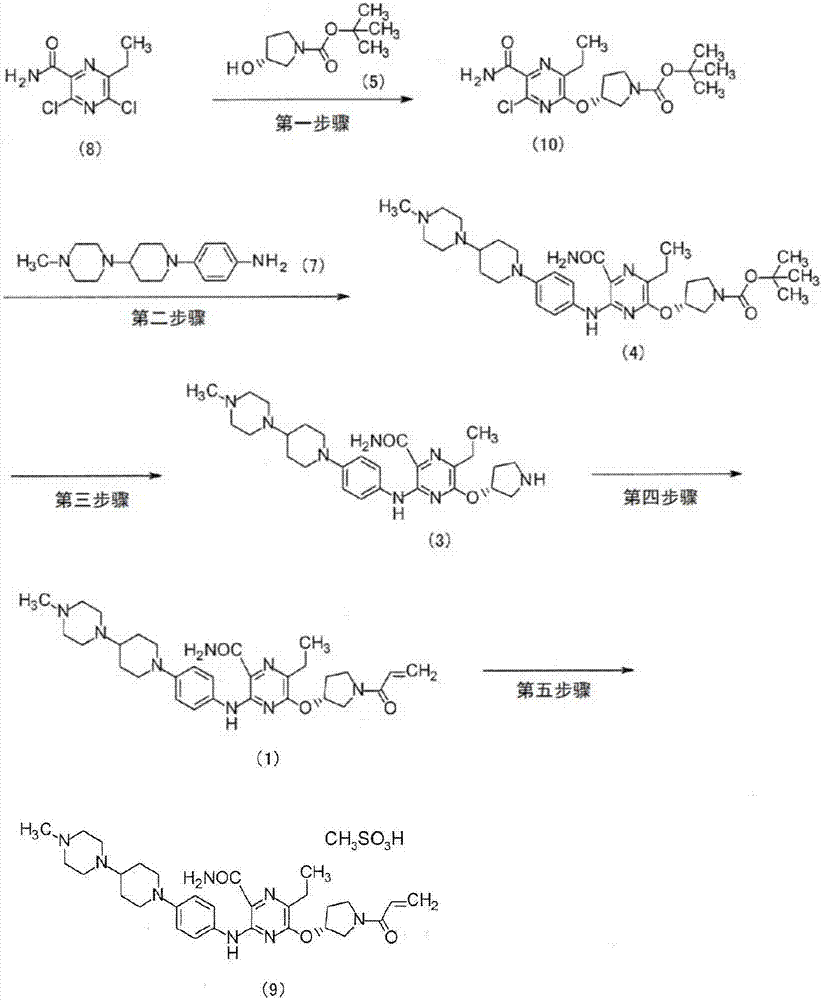
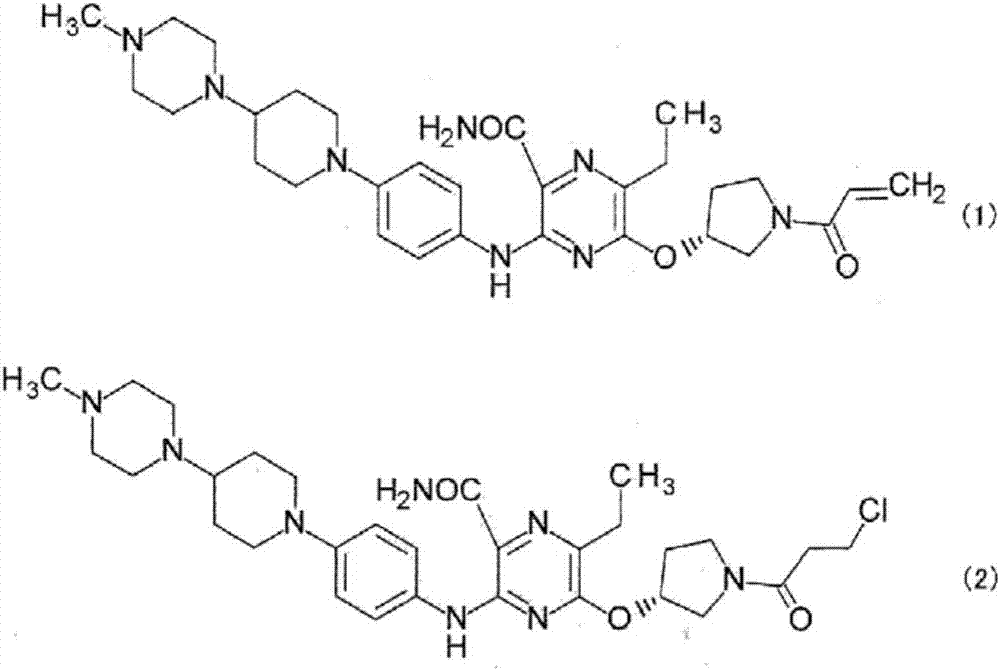
Method for producing pyrazine carboxamide compound, and synthetic intermediate thereof
A manufacturing method and compound technology, which can be used in organic chemistry, drug combination, anti-tumor drugs, etc., and can solve problems such as low yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0077] The first step 5-chloro-6-ethyl-3-{4-[4-(4-methylpiperazin-1-yl)piperidin-1-yl]anilino}pyrazine-2-carboxamide( Synthesis of compound (6))
[0078] Add 3,5-dichloro-6-ethylpyrazine-2-carboxamide (13.0kg), 4-[4-(4-methylpiperazin-1-yl)piperidin-1-yl]aniline ( 17.8kg), N,N-diisopropylethylamine (15.3kg) in 2-butanol (105.0kg) solution was stirred at 80℃~85℃ for 1 hour. After adding seed crystals (2.6 g) of compound (6), the reaction was performed at 84°C to 88°C for 15 hours. After aging for 24 hours at 22°C to 30°C, the solid was filtered and washed with 2-butanol. Dry under reduced pressure to obtain 5-chloro-6-ethyl-3-{4-[4-(4-methylpiperazin-1-yl)piperidin-1-yl]anilino}pyrazine as a solid -2-carboxamide (21.7 kg). It should be noted that the seed crystals can be produced by the following method to obtain compound (6), that is, after cooling to room temperature under the same reaction conditions without adding seed crystals, the solids are filtered, and 2-butanol Afte...
Embodiment 2
[0104] The first step 5-chloro-6-ethyl-3-{4-[4-(4-methylpiperazin-1-yl)piperidin-1-yl]anilino}pyrazine-2-carboxamide( Synthesis of compound (6))
[0105] Add 3,5-dichloro-6-ethylpyrazine-2-carboxamide (34.0kg), 4-[4-(4-methylpiperazin-1-yl)piperidin-1-yl]aniline ( 44.5kg), N,N-diisopropylethylamine (39.9kg), and 2-butanol (274.7kg) were stirred at 83°C to 86°C for 1 hour. After adding seed crystals (7 g) of compound (6), the reaction was carried out at 85°C to 86°C for 20 hours. A mixed solvent of 2-butanol (82.4 kg) and dimethyl sulfoxide (486.2 kg) was added, stirred at 84°C to 85°C for 2 hours, cooled, and stirred at 20°C to 30°C for 19 hours. The solid was filtered out and washed with dimethyl sulfoxide and 2-butanol. Dry under reduced pressure to obtain 5-chloro-6-ethyl-3-{4-[4-(4-methylpiperazin-1-yl)piperidin-1-yl]anilino}pyrazine as a solid -2-carboxamide (55.7 kg).
[0106] The second step (3R)-3-{[5-carbamoyl-3-ethyl-6-({4-[4-(4-methylpiperazin-1-yl)piperidin-1-yl] ...
reference example 1
[0119] The first step (3R)-3-[(5-carbamoyl-6-chloro-3-ethylpyrazin-2-yl)oxy]pyrrolidine-1-carboxylic acid tert-butyl ester (Compound (10) )Synthesis
[0120] In the mixture of (3R)-3-hydroxypyrrolidine-1-carboxylic acid tert-butyl ester (2600mg) and N,N-dimethylformamide (60mL), add 55% oily sodium hydride (540mg ), stirring for 1 hour. 3,5-Dichloro-6-ethylpyrazine-2-carboxamide (3000 mg) was added to the reaction solution under ice cooling, and the mixture was further stirred for 30 minutes. The reaction liquid was poured into ice water, and then extracted with ethyl acetate. After the organic layer was washed with saturated brine, it was dried with anhydrous magnesium sulfate. The solvent was distilled off under reduced pressure, and the obtained residue was purified by silica gel column chromatography (hexane / ethyl acetate). The obtained solid was washed with diisopropyl ether to obtain (3R)-3-[(5-carbamoyl-6-chloro-3-ethylpyrazin-2-yl)oxy] as a solid Tert-Butyl pyrrolidi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com