Preparation method of Cu/Pd alloy modified TiO2 catalyst for reductive dechlorination materials
A catalyst and alloy technology, applied in the field of material preparation and environment, to achieve the effect of reducing the amount of precious metals and good dechlorination effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
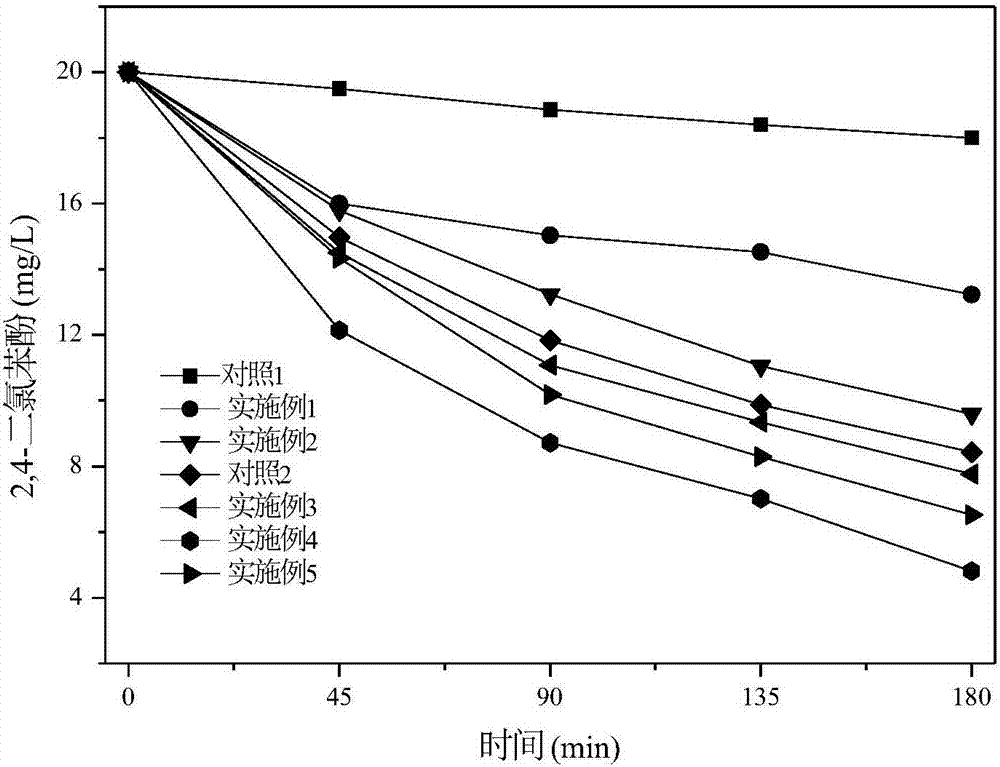
Image
Examples
Embodiment 1
[0028] (1) Preparation of nanosheet TiO by hydrothermal method 2 catalyst
[0029] Take 1 mL of HF solution with a concentration of 40% by mass, and add it dropwise into a beaker containing 25 mL of tetrabutyl titanate; stir for 2 hours, then transfer it to a hydrothermal kettle, and place it in an oven to raise the temperature to 180°C for 24 hours; the resulting white The solid was first washed three times with deionized water, and then washed three times with absolute ethanol, and then dried in an oven; after the solid powder was ground, it was calcined in a muffle furnace at 350°C for 2 hours, and the nano-flaky TiO was obtained after cooling. 2 catalyst;
[0030] (2) Preparation of Cu / Pd alloy co-modified nanoflake TiO by hydrothermal method 2
[0031] Take 0.088g Na 2 PdCl 4 , 0.034g CuCl 2 2H 2 O and 0.186g disodium edetate were dissolved in 100mL deionized water to form a mixed solution, and 7.98g (1) of prepared TiO was added 2 The catalyst was transferred to ...
Embodiment 2
[0034] (1) Preparation of nanosheet TiO by hydrothermal method 2 catalyst
[0035] Take 2 mL of HF solution with a concentration of 40% by mass, and add it dropwise into a beaker containing 25 mL of tetrabutyl titanate; stir for 2 hours, then transfer it to a hydrothermal kettle, place it in an oven and raise the temperature to 180°C for 24 hours; the resulting white The solid was first washed three times with deionized water, and then washed three times with absolute ethanol, and then dried in an oven; after the solid powder was ground, it was calcined in a muffle furnace at 350°C for 2 hours, and the nano-flaky TiO was obtained after cooling. 2 catalyst;
[0036] (2) Preparation of Cu / Pd alloy co-modified nanoflake TiO by hydrothermal method 2
[0037] Take 0.11g Na 2 PdCl 4 , 0.094g CuSO 4 ·5H 2 O and 0.28g of disodium edetate were dissolved in 100mL of deionized water to form a mixed solution, and 7.98g of TiO prepared in (1) was added 2 The catalyst was transferre...
Embodiment 3
[0040] (1) Preparation of nanosheet TiO by hydrothermal method 2 catalyst
[0041] Take 3 mL of HF solution with a concentration of 40% by mass, and add it dropwise into a beaker containing 25 mL of tetrabutyl titanate; stir for 2 hours, then transfer it to a hydrothermal kettle, place it in an oven and raise the temperature to 180°C for 24 hours; the resulting white The solid was first washed three times with deionized water, and then washed three times with absolute ethanol, and then dried in an oven; after the solid powder was ground, it was calcined in a muffle furnace at 350°C for 2 hours, and the nano-flaky TiO was obtained after cooling. 2 catalyst;
[0042] (2) Preparation of Cu / Pd alloy co-modified nanoflake TiO by hydrothermal method 2
[0043] Take 0.28g PdCl 2 , 0.10g CuSO 4 ·5H 2 O and 0.47g disodium citrate were dissolved in 100mL deionized water to form a mixed solution, and 7.98g (1) of TiO prepared in 2 The catalyst was transferred to an oven at 80°C an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com