Application of AMD3100 to preparation of medicine for treating and/preventing dyscrasia
A cachexia and drug technology, applied in the field of medicine, can solve problems such as undiscovered AMD3100
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Embodiment 1 The cultivation of Lewis lung cancer LLC, and the isolation and cultivation of mouse Nestin+ cells
[0045] 1.1 LLC cell culture: LLC cells (ATCC #CRL-1642) were cultured with DMEM basal medium (containing 10% fetal bovine serum) for later use.
[0046] 1.2 Isolation of mouse muscle Nestin+ cells
[0047] Take adult Nestin-GFP transgenic male mice (provided by Dr Masahiro Yamaguchi, and can also be obtained through commercial channels), under sterile conditions, completely excise bilateral gastrocnemius muscles, and quickly place them in a petri dish filled with PBS. Wash the muscle block with PBS 3 times, cut into small pieces of muscle tissue, and remove blood vessels, nerves, and connective tissue as much as possible. Use type I collagenase to digest and separate muscle cells: transfer the muscle tissue to a centrifuge tube, add type I collagenase, and place in a 37°C constant temperature shaking water bath for digestion for 30 minutes. Serum was added...
Embodiment 2
[0052] Example 2 Breeding of mice and construction of tumor cachexia model
[0053] 2.1 Raising method for mice: feed mice with conventional mouse feed (protein content 20-25wt%, fat content 5%-10wt%, crude fiber content 3-5wt%).
[0054] 2.2 Construction of mouse tumor cachexia model
[0055] Male C57BL / 6 mice (purchased from the Institute of Model Animals, Nanjing University) or Nestin-GFP transgenic mice with a weight difference of no more than 2 g at the age of 8 weeks were taken, and the mice were randomly divided into a control group and a dyscrasia group. About 4*10^6 Lewis lung cancer LLC cells were subcutaneously injected into the groin of each cachexia group mouse, and the experiment process was carried out in an ultra-clean bench, paying attention to aseptic operation. The day of inoculation is counted as day 0, then day 1, day 2, and so on. On the 7th day of tumor inoculation, it was detected that the body weight of the tumor-bearing mice began to drop, and tumor...
Embodiment 3
[0056] Example 3 Evaluation of Mice Tumor Cachexia
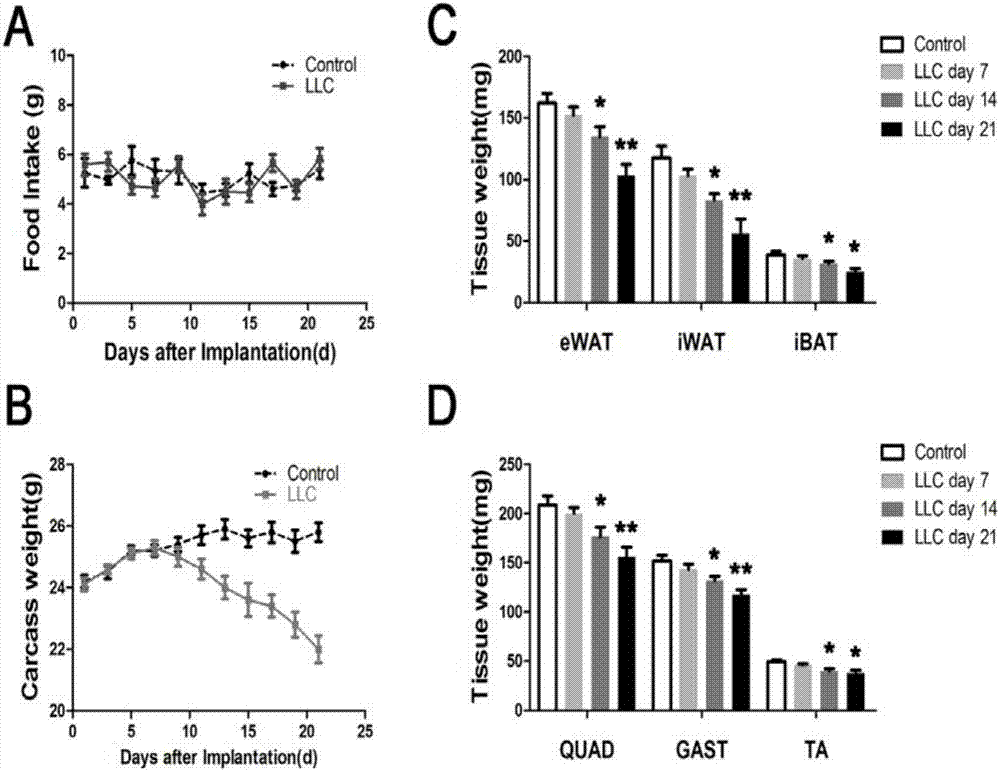
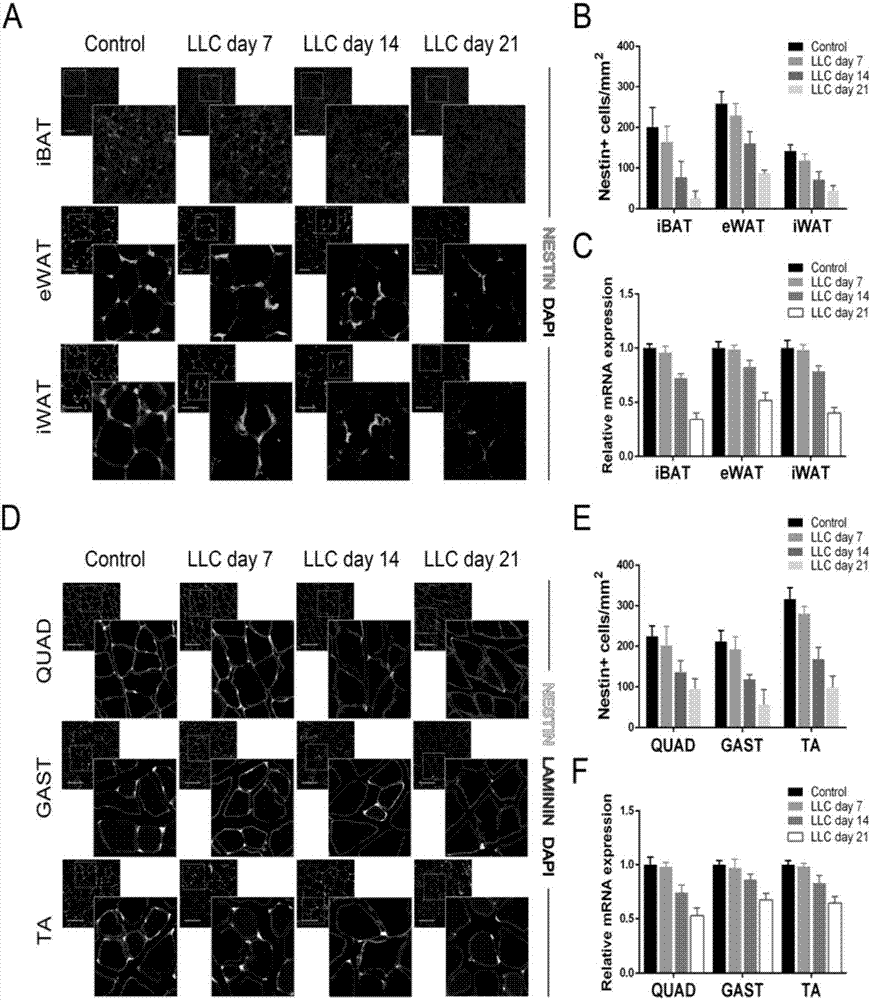
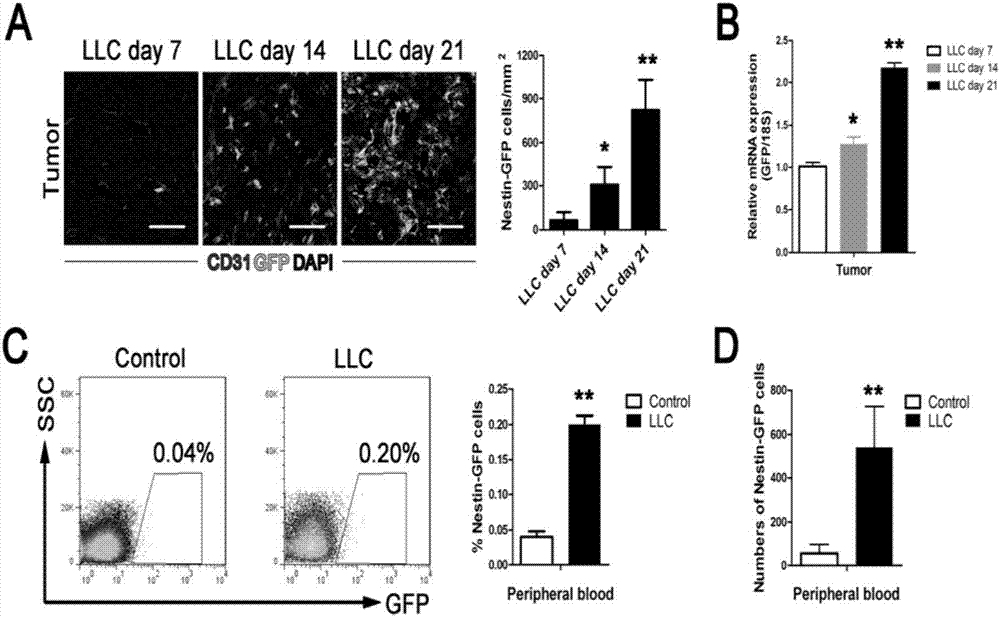
[0057] The mice in the dyscrasia group in Example 2 were observed at the same time every day after tumor inoculation, coat color, activity, body weight, food intake and tumor size, and compared with those in the control group. Every 2 days to 21 days after tumor inoculation, or on the 7th, 14th, and 21st days, the mice were killed by excessive anesthesia, the tumor tissues were removed, and the tumor-free weights of all animals were weighed; Muscle (QUAD), gastrocnemius (GAST), tibialis anterior (TA), epididymal adipose tissue (eWAT), inguinal adipose tissue (iWAT), and dorsal interscapular brown adipose tissue (iBAT) were weighed.
[0058] figure 1 Middle panel A shows the daily food intake test results of C57BL / 6 mice in the control group and dyscrasia group, panel B is the weight monitoring results of mice in the control group and dyscrasia group; panel C and panel D are adipose tissue and Weight detection of muscle tis...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com