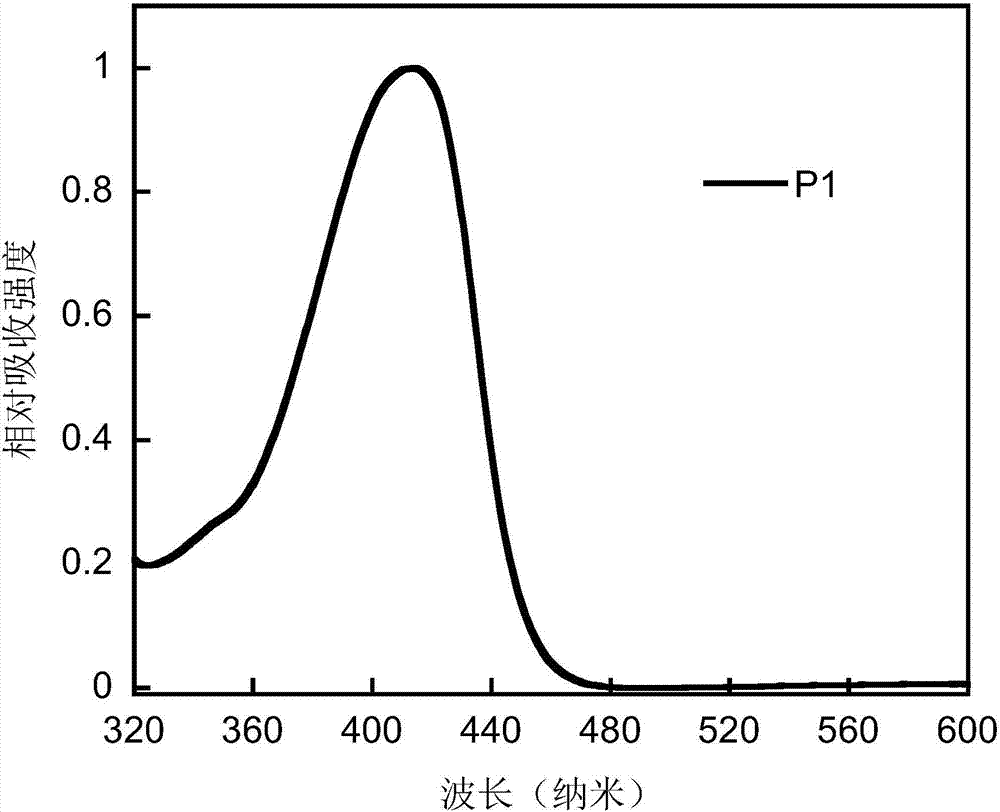
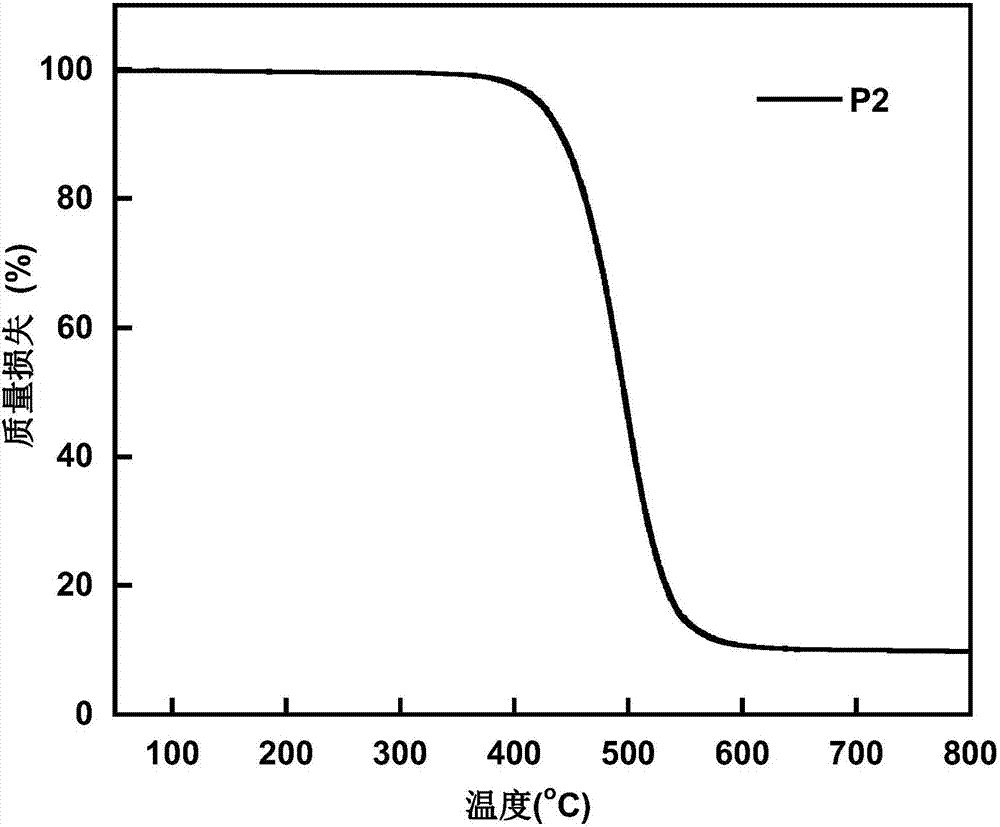
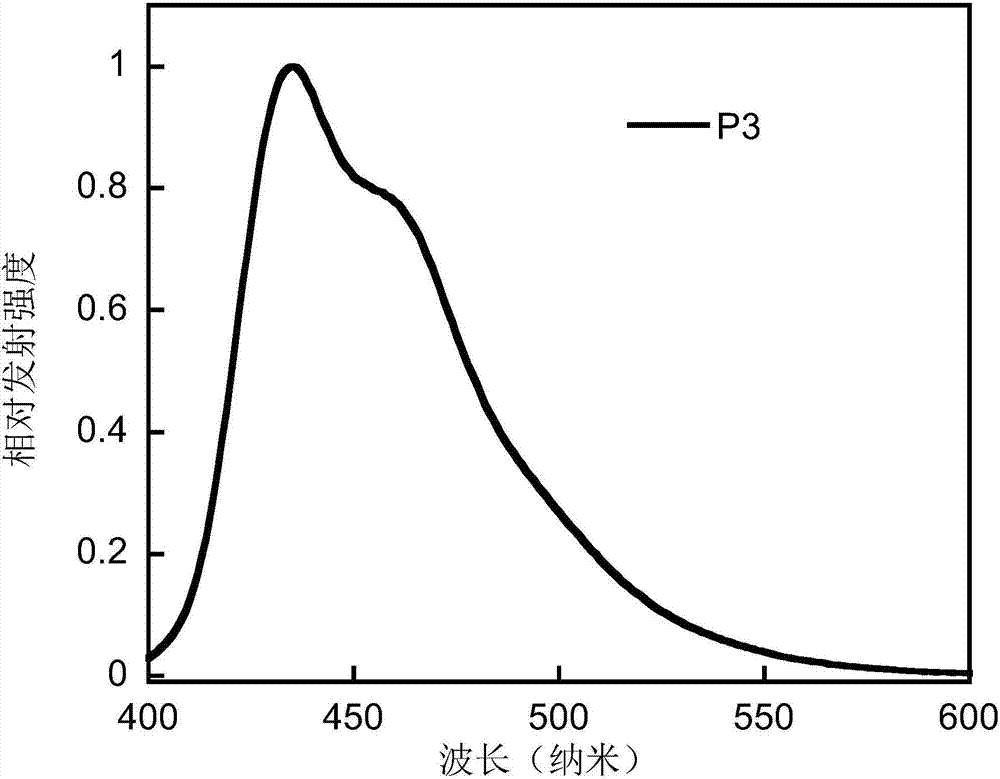
Conjugated polymer based on anthracene indenofluorene and preparation method and application thereof
A kind of conjugated polymer, anthracene indene technology, applied in the conjugated polymer based on anthracene indenofluorene unit and its preparation field
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] Preparation of 9,10-dibromo-1,5-dimethylanthracene borate
[0065] Under argon atmosphere, 9,10-dibromo-1,5-dimethylanthracene (10 g, 27.47 mmol), bisborate (20.92 g, 82.40 mmol) and potassium acetate (13.48 g, 137.33 mmol) Add it into 250 ml of 1,4-dioxane, raise the temperature to 110°C, and react for 24 hours. The reaction mixture was poured into water, extracted with ethyl acetate, and the organic layer was washed with brine and dried over anhydrous magnesium sulfate. After the solution was concentrated, a crude white solid was obtained, which was purified by silica gel column chromatography (petroleum ether / dichloromethane=3 / 1, v / v as the eluent). The product was placed in a refrigerator to obtain a white solid with a yield of 85%. 1 H NMR, 13 CNMR, MS and elemental analysis results show that the obtained compound is the target product, and the chemical reaction equation of the preparation process is as follows:
[0066]
Embodiment 2
[0068] Preparation of compound M1
[0069] Under argon atmosphere, 9,10-dibromo-1,5-dimethylanthracene borate (10 g, 21.82 mmol), sodium carbonate (11.57 g, 109.12 mmol) and 1-bromo-benzoic acid methyl ester (14.08g, 65.47mmol) was added into 250ml of toluene for complete dissolution, then tetrakistriphenylphosphine palladium (504.39mg, 436.49umol) was added, the temperature was raised to 110°C, and the reaction was carried out for 16 hours. The reaction mixture was poured into water, extracted with ethyl acetate, and the organic layer was washed with brine and dried over anhydrous magnesium sulfate. After the solution was concentrated, a crude white solid was obtained, which was purified by silica gel column chromatography (petroleum ether / dichloromethane=3 / 1, v / v as the eluent). The product was placed in a refrigerator to obtain a white solid with a yield of 85%. 1 H NMR, 13 CNMR, MS and elemental analysis results show that the obtained compound is the target product M1, a...
Embodiment 3
[0072] Preparation of Octylmagnesium Bromide
[0073] Under argon atmosphere, elemental magnesium (3.15g, 129.45mmol) and iodine (65.71mg, 258.90umol) were added to 100ml of anhydrous tetrahydrofuran, heated to 110°C, and then bromooctane (5g, 25.89mmol) was added . After two hours of reaction, the product was directly used in the next step without any treatment.
[0074]
PUM
| Property | Measurement | Unit |
|---|---|---|
| thermal decomposition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com