A copolymer luminescent material containing fluorinated side groups and its preparation method and application
A technology of luminescent materials and copolymers, which is applied in the fields of luminescent materials, chemical instruments and methods, semiconductor/solid-state device manufacturing, etc., to achieve the effect of simple synthesis method, good planarity, and large conjugate length
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Preparation of PFSO1:
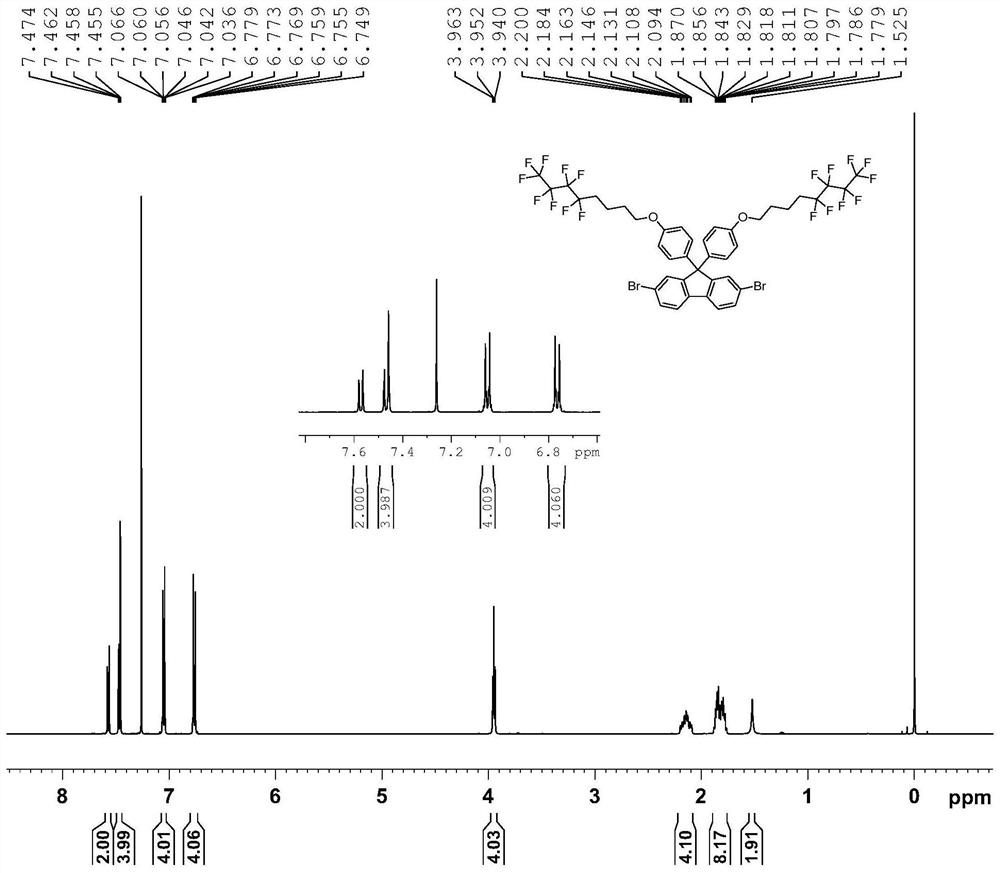
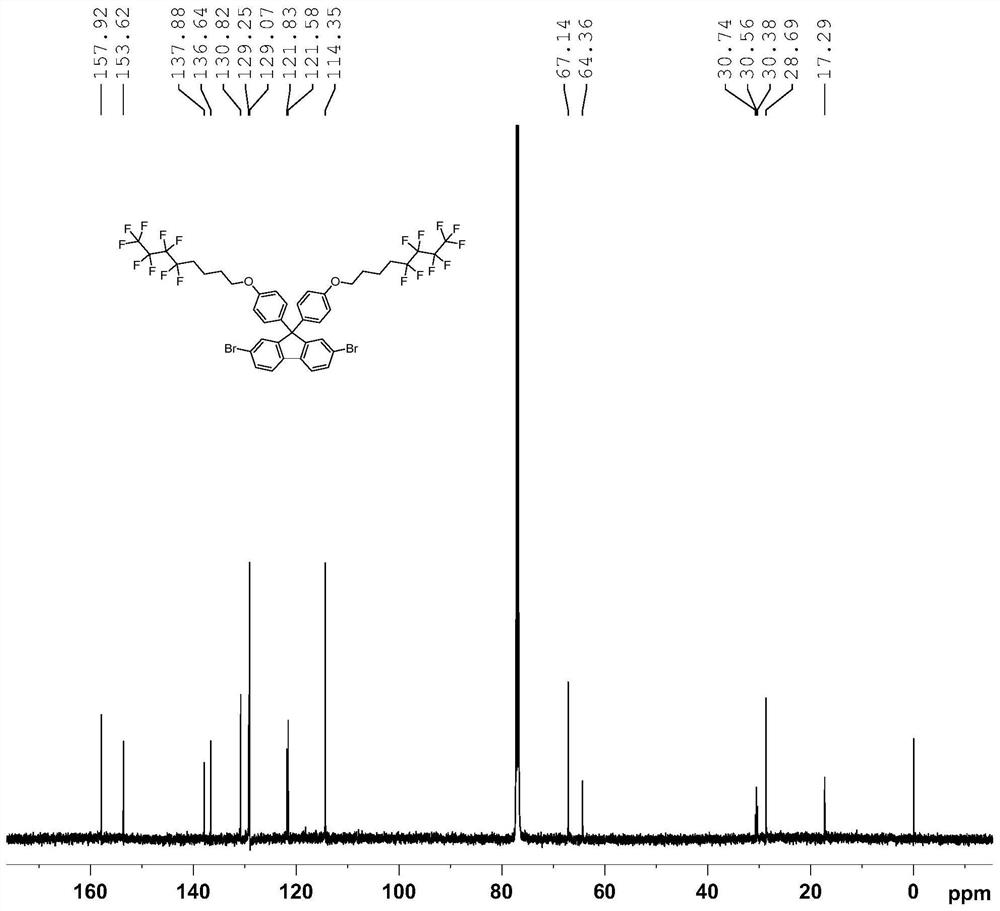
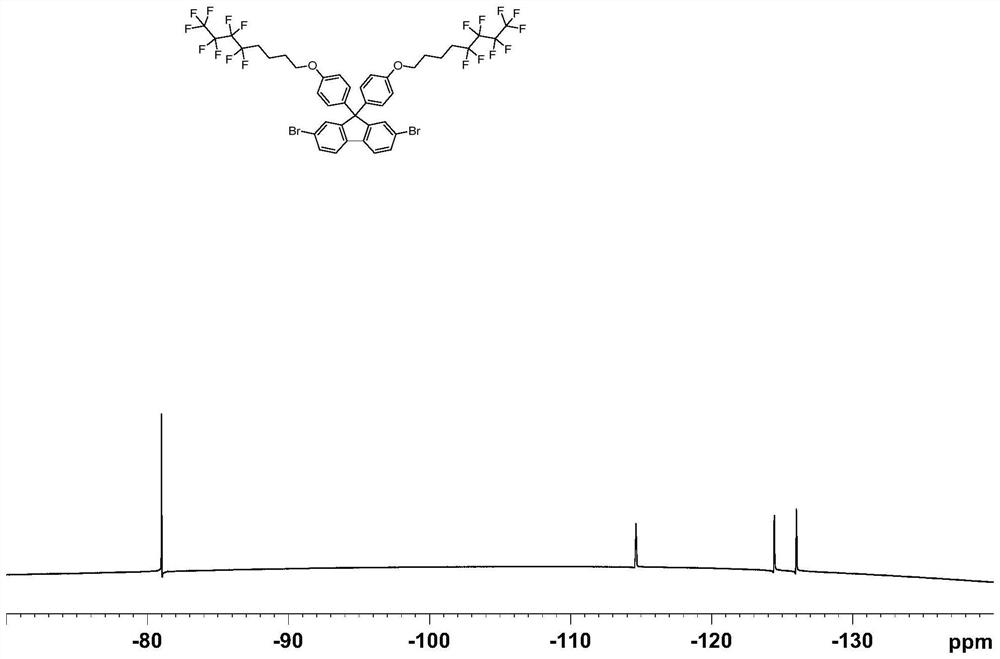
[0049] (1) Under a nitrogen atmosphere, 2,7-dibromo-(9,9-diphenylhydroxy)fluorene (2g, 3.94mol), C 8 h 8 f 9 I (3.96g, 9.84mmol), K 2 CO 3 (2.18g, 15.74mmol) and 100ml of N'N-dimethylacetamide were added to a 250ml two-necked flask, heated to 80°C for 12 hours, then returned to room temperature, extracted with ethyl acetate, washed with saturated aqueous sodium chloride solution, no dried over magnesium sulfate. After distillation under reduced pressure, the crude product was purified with a silica gel column, the eluent was a mixed solvent of dichloromethane and petroleum ether (1:5 by volume), recrystallized from ethanol, and finally a white solid was obtained as compound M1 with a yield of 80%. 1 H NMR, 13 CNMR, 18 The F NMR spectra are as follows figure 1 , figure 2 and image 3 As shown, the analysis is known as the target product. The chemical reaction equation is as follows:
[0050]
[0051](2) Under an argon atmosphere, a...
Embodiment 2
[0054] Preparation of PFSO2:
[0055] Under an argon atmosphere, add M1 (49.32mg, 46.69μmol), M5 (17.46mg, 46.69μmol), M3 (76.82mg, 140.06μmol) and M4 (150.00mg, 233.44μmol) into a 50ml two-necked bottle, and then add 8ml Trifluorotoluene was completely dissolved, pumped and ventilated three times, then quickly added palladium acetate (2.10 mg, 9.34 μmol) and tricyclohexylphosphine (5.24 mg, 18.68 μmol), pumped and ventilated three times, and then added 2ml of tetraethyl hydroxide ammonium, heated to 80°C, and reacted for 24 hours. Then add 30 mg of phenylboronic acid for capping, and after 12 hours, use 0.3 ml of bromobenzene for capping and continue the reaction for 12 hours; add the product dropwise to precipitate in methanol, stir, filter, and then dissolve the crude product in 30 mL of In toluene, use 200-300 mesh silica gel as the stationary phase, and use toluene as the eluent for column chromatography. After the solvent is concentrated under reduced pressure, it is pr...
Embodiment 3
[0058] Preparation of PFSO3:
[0059] (1) Under nitrogen atmosphere, 2,7-dibromofluorene (2.00g, 6.17mmol), C 8 h 8 f 9 I (6.2g, 15.43mmol), sodium hydroxide (24g, 1.04mol), tetrabutylammonium bromide (200mg, 0.62mmol) and 100ml of toluene were added to a 250ml two-necked flask, heated to 180°C for 12 hours, and recovered At room temperature, extract with ethyl acetate, wash with saturated aqueous sodium chloride, and dry over anhydrous magnesium sulfate. After distillation under reduced pressure, the crude product was purified with a silica gel column, the eluent was a mixed solvent of dichloromethane and petroleum ether (1:5 by volume), recrystallized from methanol, and finally a white solid was obtained as compound M6 with a yield of 67%. 1 H NMR, 13 CNMR, 18 F NMR analysis was the target product. The chemical reaction equation is as follows:
[0060]
[0061] (2) Under an argon atmosphere, add M6 (40.73mg, 46.69μmol), M5 (17.46mg, 46.69μmol), M3 (76.82mg, 140.06μ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com