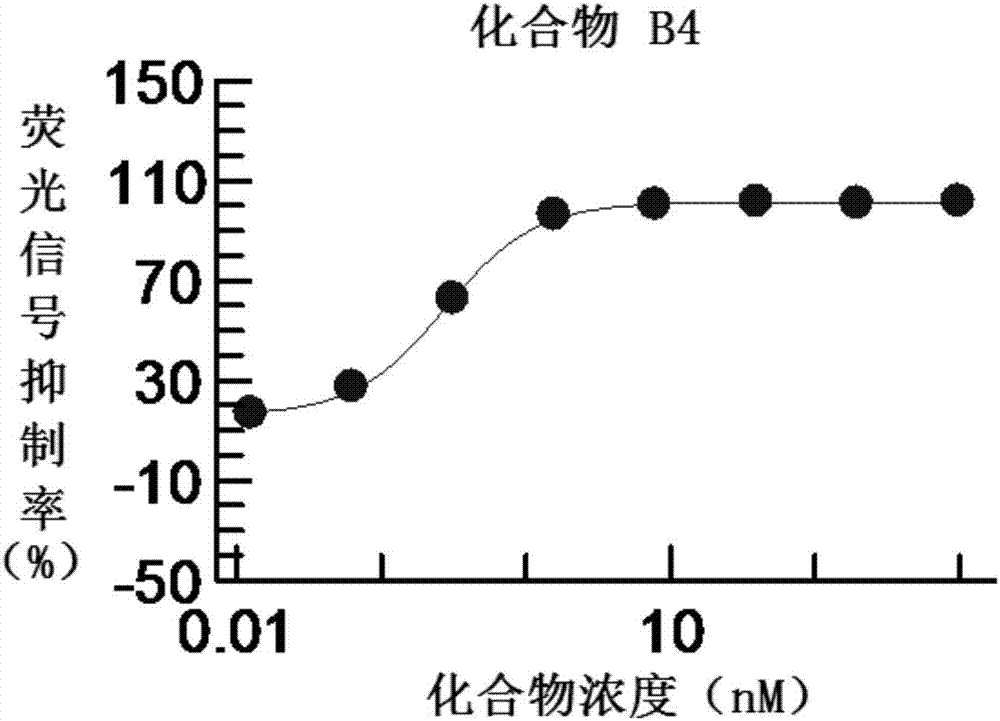
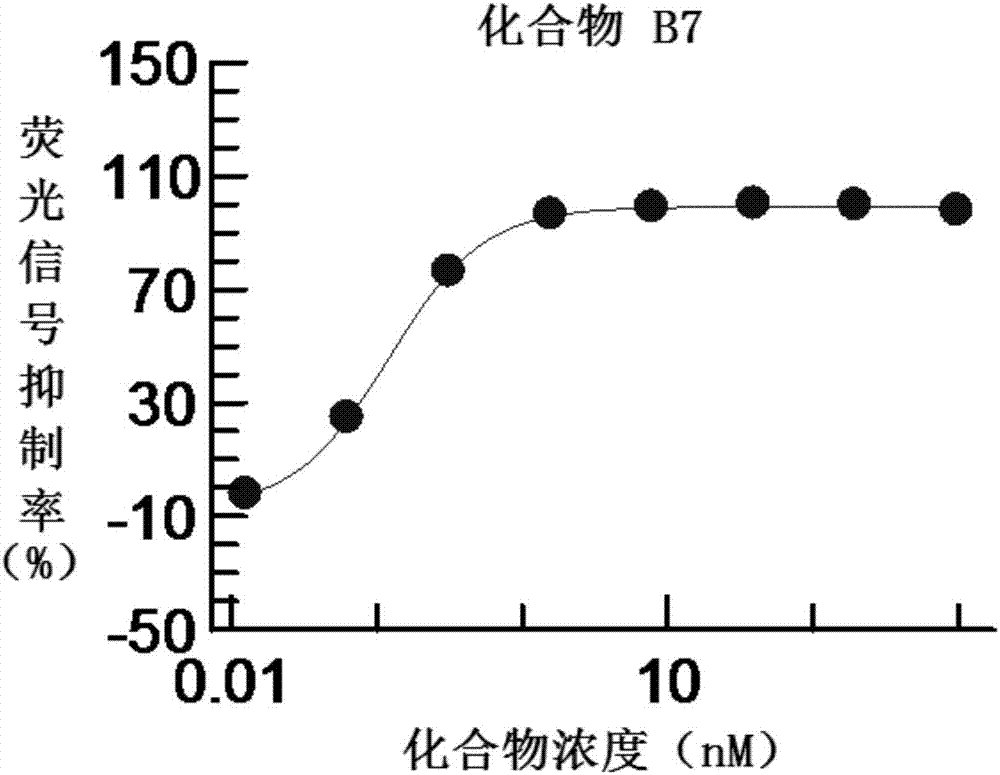
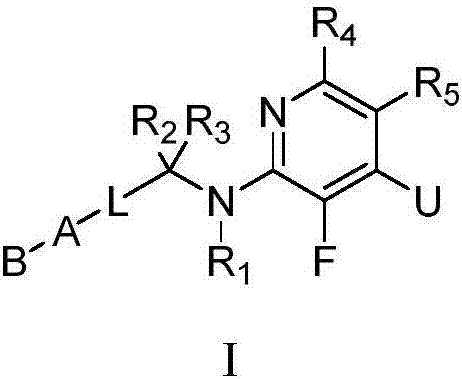
3-fluoropyridine heterocyclic compound and application thereof
A technology of heterocyclic compound, fluoropyridine, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0114] This example provides a 3-fluoropyridine heterocyclic compound and its synthesis method.
[0115] (1) Heterocyclic compound B1, which is synthesized by the following method:
[0116]
[0117] 1) Synthesis of Intermediate B1-2:
[0118] Add 20 mL of anhydrous tetrahydrofuran to a 50 mL two-neck flask, protect with nitrogen, cool to -78°C, add 2.5N LDA tetrahydrofuran solution (4.4 mL, 11 mmol), and slowly add B1-1 (1.15 g, 10 mmol). Stir at -78°C for 1 h, then slowly add triisopropyl borate (2.82 g, 15 mmol). After adding, stir at -78°C for 1.5h, then slowly rise to room temperature. 5 mL of 2N aqueous sodium hydroxide solution was added, and stirred for 10 minutes. Separate the layers, adjust the pH of the aqueous phase to 5, and extract three times with ethyl acetate. The organic phases were combined, washed once with saturated aqueous sodium chloride, dried over anhydrous sodium sulfate, and the solvent was evaporated under reduced pressure to obtain a white so...
Embodiment 2
[0126] This embodiment provides a 3-fluoropyridine heterocyclic compound B2, which is synthesized by the following method:
[0127]
[0128] 1) Synthesis of intermediate B2-2:
[0129] Dissolve compound B2-1 (5g, 34.3mmol) in acetonitrile (100mL), add NBS (18.3g, 103mmol) and benzoyl peroxide (829mg, 3.43mmol), reflux overnight, cool to room temperature, add water (200mL ), extracted with ethyl acetate (200mL×3), the organic phase was washed with saturated brine (300mL×3), dried over anhydrous sodium sulfate, filtered off sodium sulfate, spin-dried the solvent, and the residue was purified by column chromatography (petroleum ether :ethyl acetate=100:1), a yellow oil (7.45g, 97%) was obtained. 1 HNMR (400MHz, CDCl 3) δ 8.23 (d, J = 1.2 Hz, 1H), 7.56-7.53 (m, 1H), 4.45 (s, 2H).
[0130] 2) Synthesis of Intermediate B2-3:
[0131] Intermediate B2-2 (7.45g, 33.1mmol) was dissolved in DMF (50mL), NaN was added under ice-cooling 3 (8.61g, 132mmol), stirred overnight at 50°...
Embodiment 3
[0139] This embodiment provides a 3-fluoropyridine heterocyclic compound B3, which is synthesized by the following method:
[0140]
[0141] 1) Synthesis of Intermediate B3-2:
[0142] Dissolve compound B3-1 (5g, 25.4mmol) in acetonitrile (100mL), add NBS (6.8g, 38mmol) and benzoyl peroxide (100mg, 0.41mmol), reflux overnight, and then add benzoyl peroxide (50mg, 0.21mmol), and then refluxed for 12h. Cool to room temperature, concentrate and evaporate the solvent, and the residue is purified by column chromatography (petroleum ether: ethyl acetate = 100:1-50:1) to obtain a solid (4.3 g, 61%).
[0143] 2) Synthesis of Intermediate B3-3:
[0144] Intermediate B3-2 (2.3g, 8.2mmol) was dissolved in DMSO (40mL), NaN was added under ice bath 3 (637mg, 9.8mmol), stirred at room temperature for 30 minutes, added water (100mL), extracted with ethyl acetate (100mL×3), washed the organic phase with saturated brine (50mL×3), dried over anhydrous sodium sulfate, filtered off Sodium ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com