Method for measuring chromatographic purity of difluprednate
A technology for difluprednate and a determination method, which is applied in the directions of measuring devices, instruments, scientific instruments, etc., can solve the problems of complicated mobile phase preparation, unsatisfactory separation effect, low number of theoretical plates, etc., and achieves ideal separation effect, Good peak shape, simple preparation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
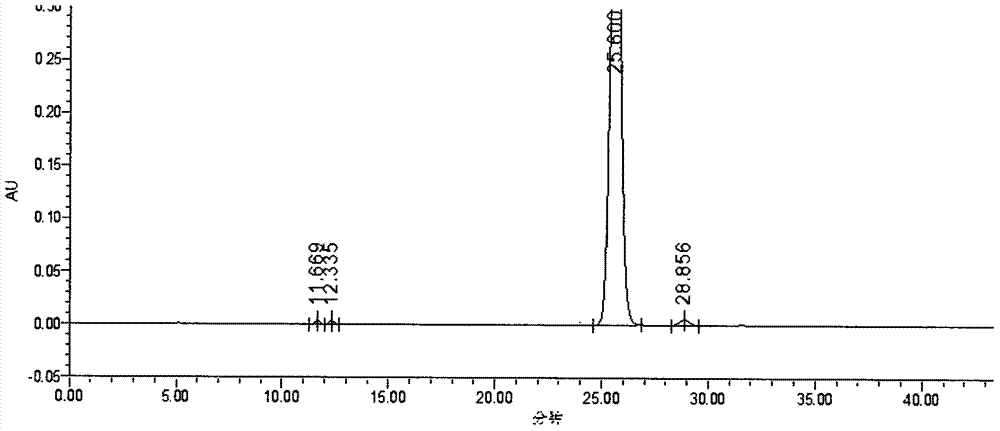
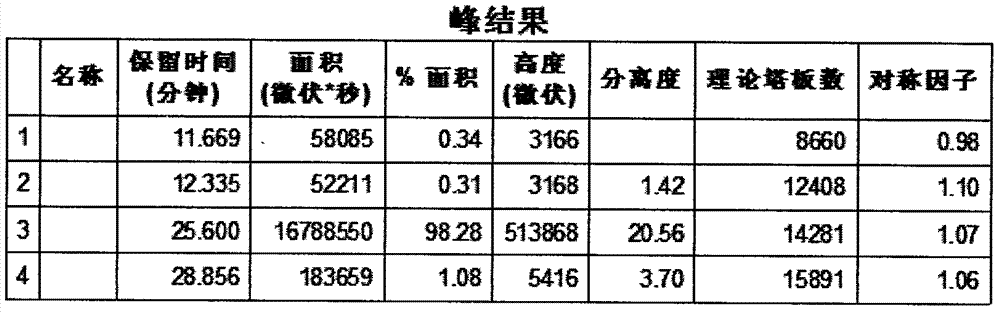
[0033] Instrument: Waters 2695 (2487 ultraviolet-visible light detector, Empower 3 chromatographic workstation), accurately measure 15 μ L of the test solution and inject it into the chromatograph, using methanol-water ratio of 68:32 as mobile phase, flow rate of 0.9ml / min, detection The wavelength is 240nm, record the chromatogram to 2 times the retention time of the main peak of difluprednate, and calculate the chromatographic purity by adjusting the appropriate instrument integration parameters and the area normalization method. The detection result shows that under this chromatographic condition, the peak eluting time of difluprednate is moderate, about 25min, the peak shape is good, see the attached figure 1 , the resolution is 20.56, see attached figure 2 . And when using methanol-water (50:50) as the mobile phase, there is no peak within 60min, the main peak will appear in the next sample, and its baseline is unstable, fluctuating from time to time, and the peak sha...
Embodiment 2
[0035] Precisely measure 15 μL of the test solution and inject it into the chromatograph, select the following chromatographic conditions for detection: use a C18 chromatographic column, column temperature 35 ° C, methanol-water ratio of 68:32 as mobile phase, flow rate of 1.0ml / min, The detection wavelength is 240nm, and the chromatogram is recorded to 2 times the retention time of the main peak of difluprednate, and the chromatographic purity is calculated by adjusting the appropriate instrument integration parameters and the area normalization method. The detection result shows that under this chromatographic condition, the peak time of difluprednate is moderate, and the peak shape is good, see the attached Figure 4 , good resolution, see attached Figure 5 .
Embodiment 3
[0037] Accurately measure 15 μ L of the test solution and inject it into the chromatograph, select the following chromatographic conditions for detection: adopt a C18 chromatographic column, column temperature 35°C, methanol-water (60:40) as mobile phase, flow rate of 1.0ml / min, The detection wavelength is 240nm, and the chromatogram is recorded to 2 times the retention time of the main peak of difluprednate, and the chromatographic purity is calculated by adjusting the appropriate instrument integration parameters and the area normalization method. The detection result shows that under this chromatographic condition, the peak time of difluprednate is moderate, and the peak shape is good, see the attached Image 6 , good resolution, see attached Figure 7 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com