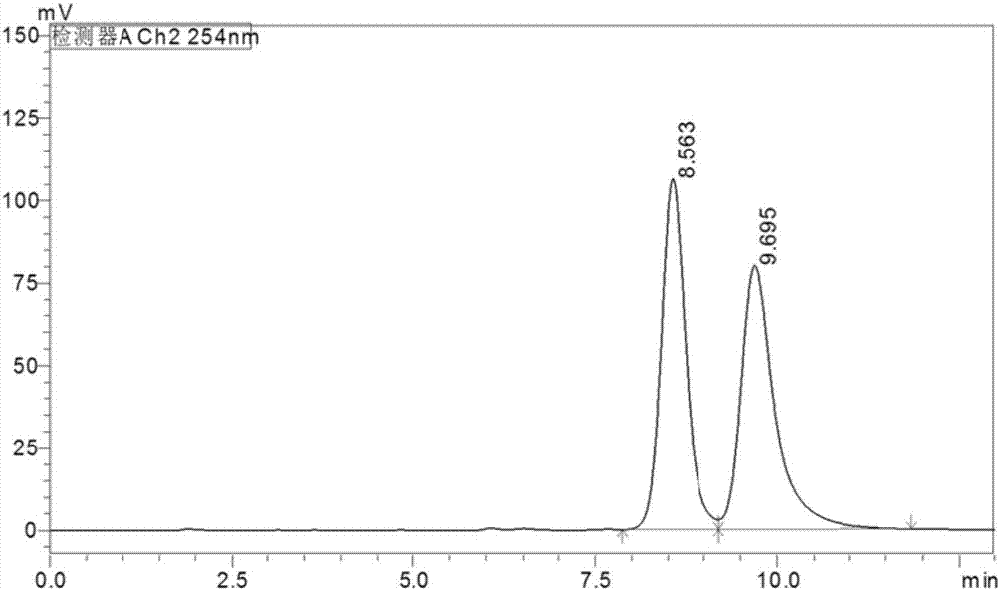
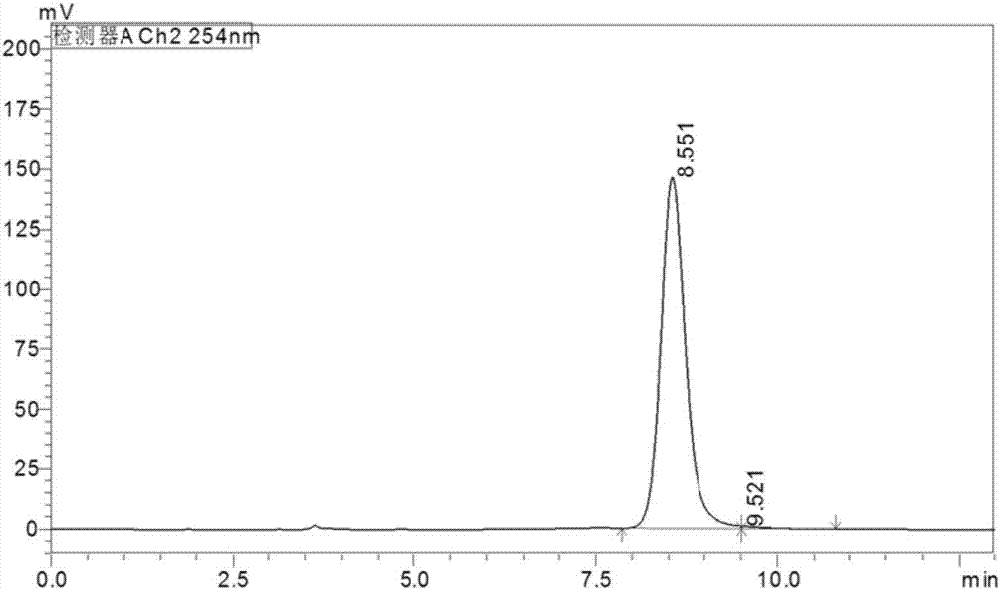
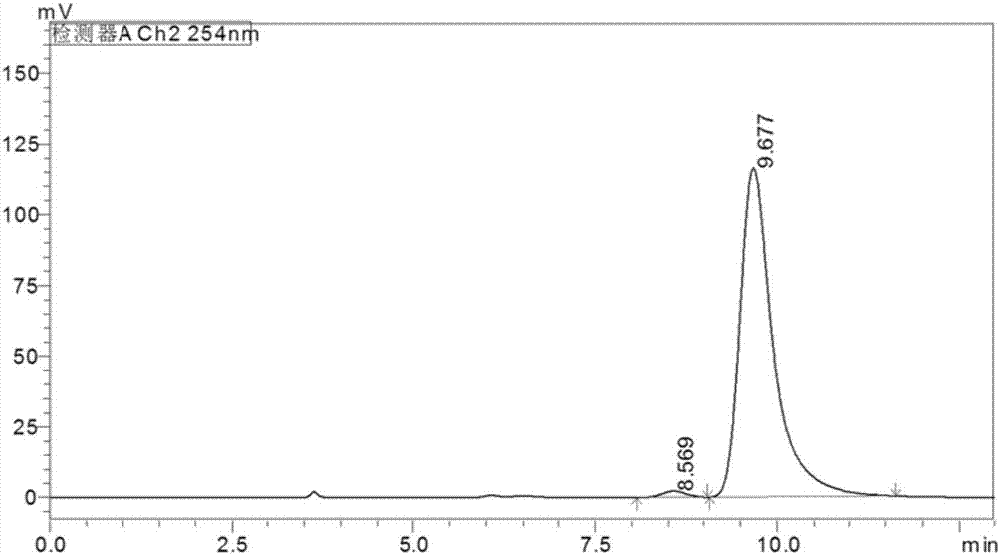
Indoleamine2,3-dioxygenase inhibitor, as well as preparation method and application thereof
A technology selected from, alkyl, and applied in the field of drug development, which can solve the problems of limited improvement, unfavorable clinical development, and poor exposure.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0169] (Z)-N 1 -(2-((4-(N-(3-bromo-4-fluorophenyl)-N'-hydroxycarbimidate)-1,2,5-oxadiazole- 3-yl)amino)ethyl)oxalamide (1)
[0170]
[0171] Step 1: N 1 -(2-((4-(4-(3-Bromo-4-fluorophenyl)-5-carbonyl-4,5-dihydro-1,2,4-oxadiazole-3- (Base)-1,2,5-oxadiazol-3-yl)amino)ethyl)oxalamide 1m.
[0172] Put 3-(4-((2-aminoethyl)amino)-1,2,5-oxadiazol-3-yl)-4-(3-bromo-4-fluorophenyl) in a 100mL single-neck bottle -1,2,4-oxadiazole-5(4H)-one 1l (300mg, 0.78mmol), oxalamide (138mg, 1.56mmol) dissolved in N,N-dimethylformamide (8mL), add O-benzotriazole-N,N,N',N'-tetramethylurea tetrafluoroborate (375.6mg, 1.17mmol), then add N,N-diisopropylethylamine (0.5mL, 2.34 mmol), react at room temperature for 2 hours, add water (50mL), solid precipitate, filter, dry solid to obtain N 1 -(2-((4-(4-(3-Bromo-4-fluorophenyl)-5-carbonyl-4,5-dihydro-1,2,4-oxadiazol-3-yl)-1 ,2,5-oxadiazol-3-yl)amino)ethyl)oxalamide 1m (105mg), the yield is 32.0%.
[0173] MS m / z (ESI): 456.0, 458.0 (M, M+2).
[0174] Step 2:...
Embodiment 2
[0179] (Z)-N 1 -(2-((4-(N-(3-bromo-4-fluorophenyl)-N'-hydroxycarbimidate)-1,2,5-oxadiazole- 3-yl)amino)ethyl)-N 2 -Methyloxalamide (2)
[0180]
[0181] Step 1: Methyl 2-((2-((4-(4-(3-bromo-4-fluorophenyl)-5-carbonyl-4,5-dihydro-1,2,4-oxadiazole - 3-yl)-1,2,5-oxadiazol-3-yl)amino)ethyl)amino)-2-carbonyl acetate 2b.
[0182] Put 3-(4-((2-aminoethyl)amino)-1,2,5-oxadiazol-3-yl)-4-(3-bromo-4-fluorophenyl) in a 100mL single-neck bottle -1,2,4-oxadiazole-5(4H)-one 1l (385mg, 1.0mmol), dimethyl oxalate (141.6mg, 1.2mmol) dissolved in methanol (15mL), add sodium methoxide (130mg, 2.4 mmol), react at room temperature overnight, LC-MS monitors the complete conversion of the raw materials, and stop the reaction. Saturated ammonium chloride solution (30mL) was added, extracted with ethyl acetate (50mL x 2), the combined organic phases were washed with saturated sodium chloride (50mL), dried over anhydrous sodium sulfate, filtered, and the filtrate was concentrated to obtain methyl 2- (...
Embodiment 3
[0189] (Z)-N 1 -(2-((4-(N-(3-bromo-4-fluorophenyl)-N'-hydroxycarbimidate)-1,2,5-oxadiazole- 3-yl)amino)ethyl)-N 2 -Ethyloxalamide (3)
[0190]
[0191] Step 1: N 1 -(2-((4-(4-(3-Bromo-4-fluorophenyl)-5-carbonyl-4,5-dihydro-1,2,4-oxadiazole-3- (Yl)-1,2,5-oxadiazol-3-yl)amino)ethyl)-N 2 -Ethyloxalamide 3b.
[0192] Methyl 2-((2-((4-(4-(3-bromo-4-fluorophenyl)-5-carbonyl-4,5-dihydro-1,2,4- Oxadiazol-3-yl)-1,2,5-oxadiazol-3-yl)amino)ethyl)amino)-2-carbonyl acetate (240mg, 0.51mmol) dissolved in methanol (15mL), 1M ethylamine solution (2 mL) was added to the above solution, and reacted at room temperature for 3 hours. LC-MS monitored the complete conversion of the raw materials, the reaction was stopped, water (30 mL) was added, and the mixture was extracted with ethyl acetate (30 mL x 2). The combined organic phases were washed with saturated sodium chloride (30 mL), dried over anhydrous sodium sulfate, filtered, and the filtrate Concentrate, get N 1 -(2-((4-(4-(3-Bromo-4-fluorop...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com