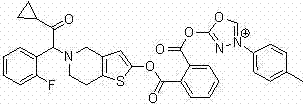
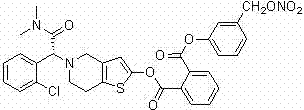
Thiophene pyridine derivative as well as preparation method and application thereof
A compound and technology of general formula, applied in the field of thiophenepyridine derivatives and their preparation, can solve the problems of cardiovascular events, increased mortality, serious aftermath, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
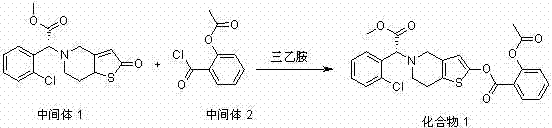
[0110] Embodiment 1: the preparation method of compound 1, its synthetic route is as follows:
[0111]
[0112] The levorotatory form of Intermediate 1 (10 mmol) was dissolved in 20 mL of anhydrous dichloromethane, and added dropwise to the anhydrous dichloromethane in which Intermediate 2 (11 mmol) and triethylamine (15 mmol) were dissolved at about 0 °C. After completion, the temperature was controlled at about 0°C and stirring was continued for 2 hours. The reaction solution was poured into 60 mL of ice water, extracted with ethyl acetate (100 mL × 3), the organic phases were combined, the organic phases were washed with saturated sodium chloride solution, dried over anhydrous sodium sulfate, concentrated and evaporated to dryness, and the target was obtained by flash column chromatography The product compound 1.
Embodiment 2
[0113] Embodiment 2: The preparation method of compound 2 is as follows:
[0114] It was prepared according to the method of Example 1, except that the intermediate 1 levorotate was replaced with the intermediate 1 dextrorotor.
Embodiment 3
[0115] Embodiment 3: The preparation method of compound 3 is as follows:
[0116] Prepared according to the method of Example 1, except that the intermediate 1 levorotate is replaced by the intermediate 1 racemate.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com