Preparation method for 4-methoxy-1,3-phthalic acid
A technology of phthalic acid and methoxy, which is applied in the field of large-scale production of 4-methoxy-1,3-benzenedicarboxylic acid, can solve problems such as environmental hazards, equipment corrosion, and difficult completion, and reduce environmental pollution , less investment in equipment and low synthesis cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
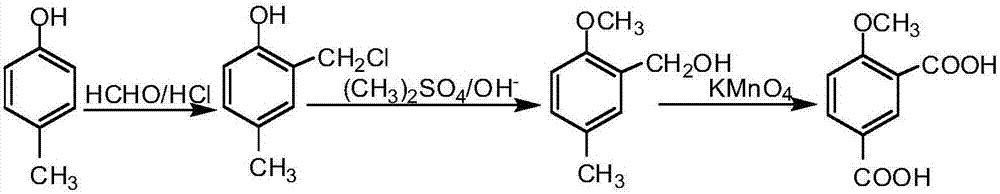
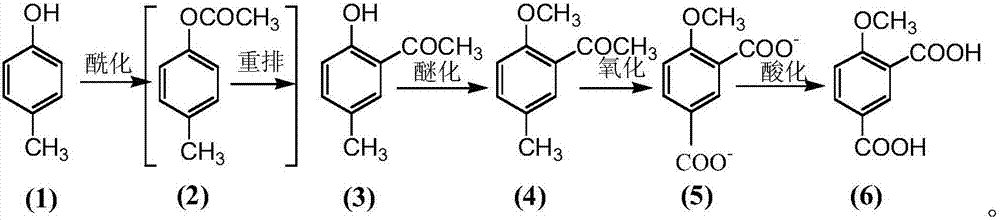
[0025] The present embodiment adopts p-cresol (1) as starting raw material, obtains p-methylphenyl acetate (2) through esterification reaction, obtains 5-methyl-2-hydroxyacetophenone (3) through Fries rearrangement reaction ), through methylation reaction to obtain 5-methyl-2-methoxyacetophenone (4), through oxidation reaction to obtain 4-methoxy-1,3-phthalic acid salt, through acidification reaction to obtain 4 -Methoxy-1,3-phthalic acid (6).
[0026] Reaction 1:
[0027]
[0028] Synthesis of 5-methyl-2-hydroxyacetophenone (3)
[0029] Put 20g of p-cresol in a 250mL round bottom flask, add 18mL of acetic anhydride and 30g of aluminum trichloride, and react at 110°C for 8h. Add 100 mL of hydrochloric acid. Extract with dichloromethane. 27.5 g of crude product 5-methyl-2-hydroxyacetophenone (3) was obtained, with a yield of 99.0%, which could be directly used in the next step without purification.
[0030] Reagents in this step include carboxylic acids, acid halides an...
Embodiment 2
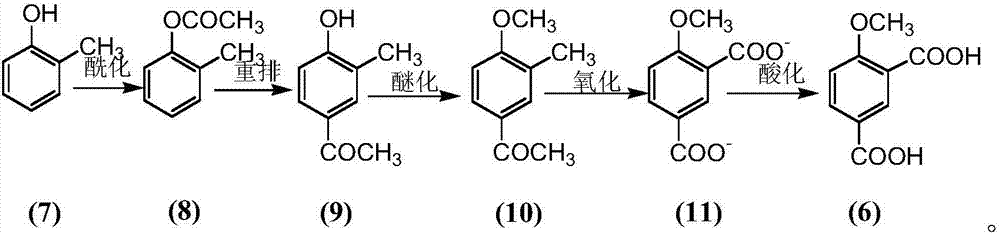
[0038] Adopt o-cresol as starting material (7), obtain o-methyl phenyl acetate (8) through esterification, obtain 3-methyl-4-hydroxyacetophenone (9) through Fries rearrangement reaction, obtain through The methylation reaction gives 4-methoxy-3-methylacetophenone (10), the oxidation reaction gives 4-methoxy-1,3-benzenedicarboxylate (11), and the acidification reaction gives 4 -Oxymethyl-1,3-phthalic acid.
[0039] Reaction 2:
[0040]
[0041] Synthesis of 3-methyl-4-hydroxyacetophenone (9)
[0042] Put 20g of o-cresol in a 250mL round bottom flask, add 18mL of acetic anhydride and 30g of aluminum trichloride, and react at 160°C for 8h. Add 100 mL of hydrochloric acid. Extract with dichloromethane. 26.5 g of crude product 3-methyl-4-hydroxyacetophenone (9) was obtained, with a yield of 95.0%, which could be directly used in the next step without purification.
[0043] The reaction reagent used in this step includes carboxylic acid, carboxylic acid ester, acid chloride ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com