Zeolite-like imidazate framework material and preparation method thereof
A technology of zeolite imidazolate and framework materials, applied in chemical instruments and methods, 2/12 group organic compounds without C-metal bonds, zinc organic compounds, etc., can solve the problems of by-products and amorphous crystals, and achieve The effect of high crystallinity, low overall cost, and large specific surface area
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] A preparation method for a zeolite-like imidazolate framework material, comprising the following steps:
[0027] (1) Metal salt Zn(NO 3 ) 2 ·6H 2 O (1.49g, 5.01mmol), imidazole derivative 2-methylimidazole (2.47g, 29.96mmol) and quaternary ammonium salt Eu 4 NBr (6.44g, 19.98mmol) was mixed, stirred and reacted for 30min at 60°C to obtain a homogeneous solution; Zn(NO 3 ) 2 ·6H 2 O: 2-methylimidazole: Eu 4 NBr molar ratio = 1:6:4;
[0028] (2) After the homogeneous solution is cooled to room temperature, add deionized water (the amount is 5 times the volume of the homogeneous solution) to obtain a suspension;
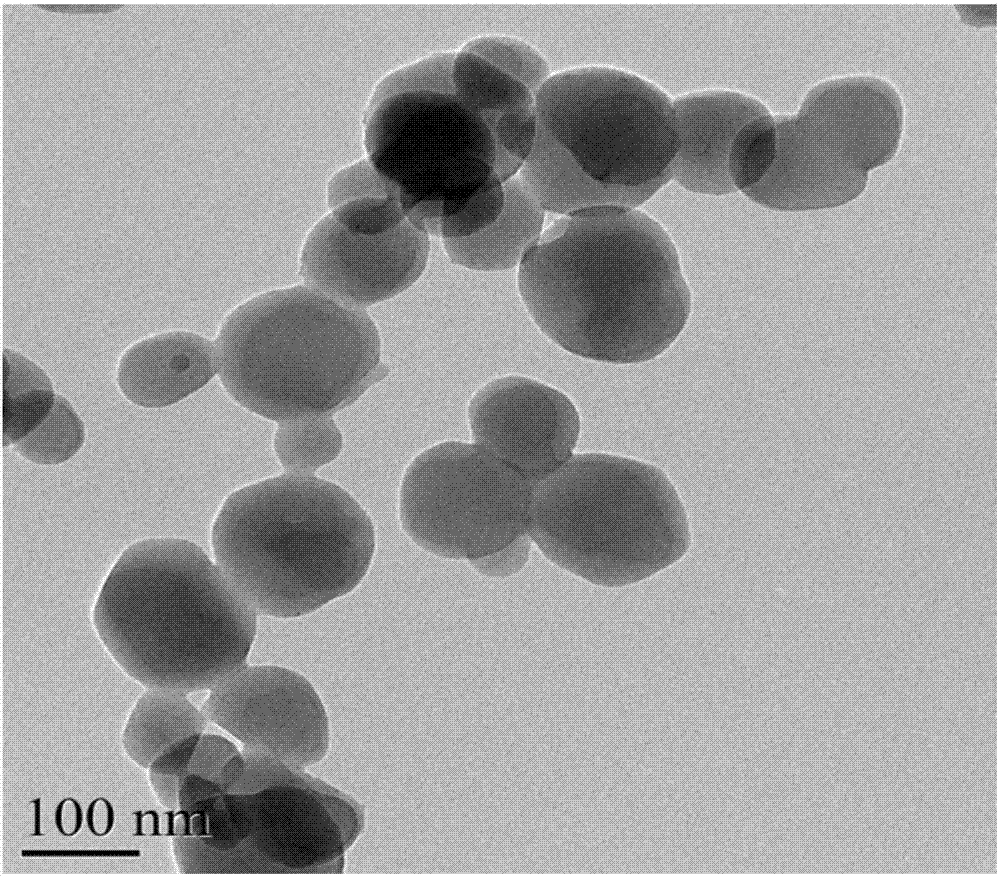
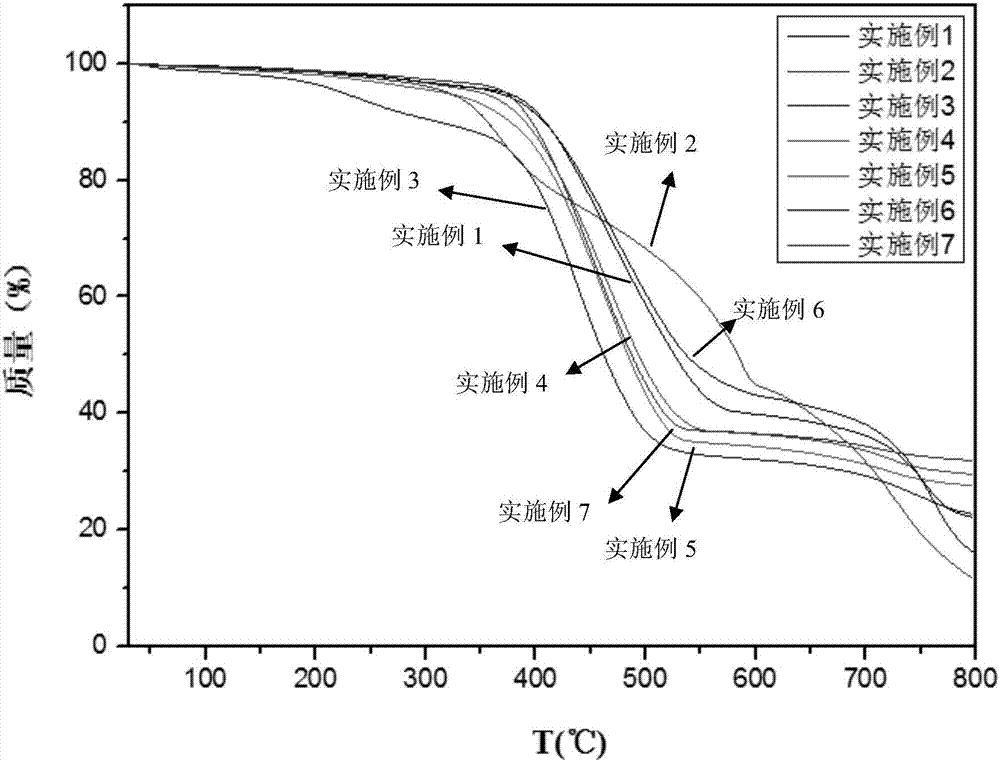
[0029] (3) Centrifuge the suspension, wash with water and ethanol, and dry at 110° C. for 12 hours to obtain the zeolite-like imidazolate framework material. The performance test results of the zeolite-like imidazolate framework material prepared in this example are shown in Table 1.
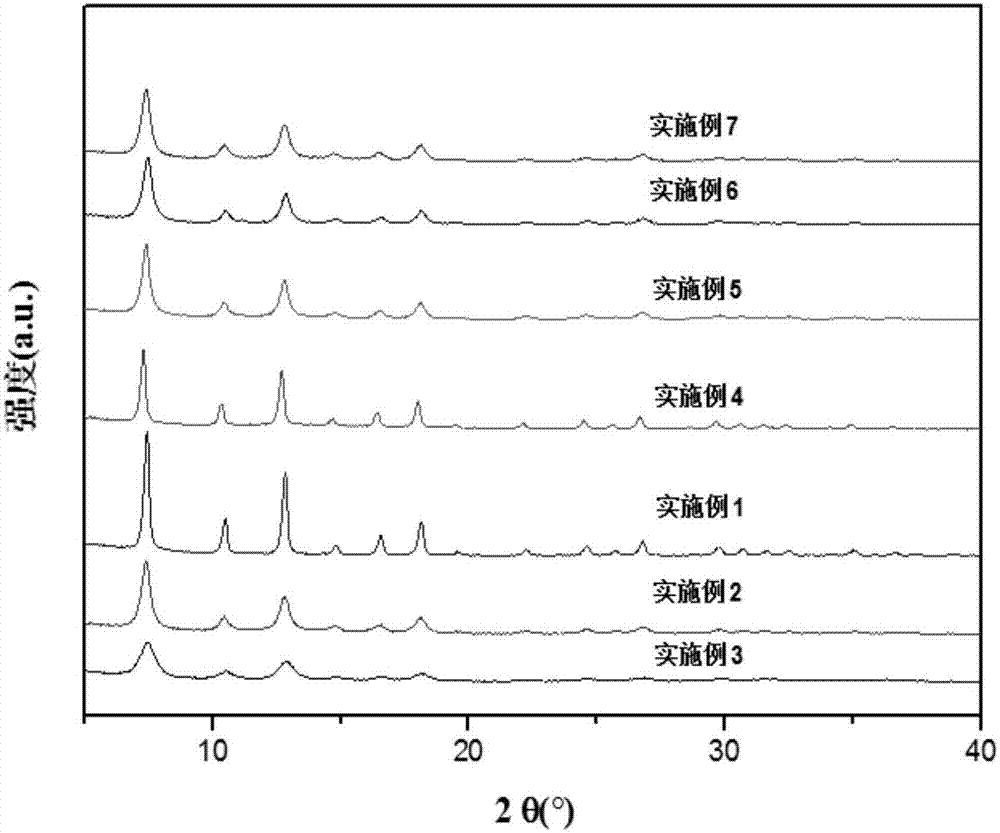
[0030] The X-ray diffraction spectrum of the zeolite imidazolate fram...
Embodiment 2
[0032] A preparation method for a zeolite-like imidazolate framework material, comprising the following steps:
[0033] (1) Metal salt Zn(OAc) 2 2H 2 O (0.63g, 2.89mmol), imidazole derivative 2-methylimidazole (0.95g, 11.57mmol) and quaternary ammonium salt ChCl (1.07g, 7.69mmol) were mixed, stirred and reacted at 60°C for 30min to obtain a homogeneous solution ; Zn(OAc) 2 2H 2 O: 2-methylimidazole: ChCl molar ratio = 1: 4: (8 / 3);
[0034] (2) After the homogeneous solution is cooled to room temperature, add deionized water (the amount is 5 times the volume of the homogeneous solution) to obtain a suspension;
[0035] (3) Centrifuge the suspension, wash with water and ethanol, and dry at 110° C. for 12 hours to obtain the zeolite-like imidazolate framework material. The performance test results of the zeolite-like imidazolate framework material prepared in this example are shown in Table 1.
[0036] The X-ray diffraction pattern of the zeolite-like imidazolate framework ...
Embodiment 3
[0038] A preparation method for a zeolite-like imidazolate framework material, comprising the following steps:
[0039] (1) Metal salt Zn(NO 3 ) 2 ·6H 2 O (3.33g, 11.19mmol), imidazole derivative 2-ethylimidazole (2.15g, 22.41mmol) and quaternary ammonium salt Eu 4 NBr (4.81g, 14.92mmol) was mixed, stirred and reacted at 60°C for 30min to obtain a homogeneous solution; Zn(NO 3 ) 2 ·6H 2 O: 2-ethylimidazole: Eu 4 NBr molar ratio=1:2:(4 / 3);
[0040] (2) After the homogeneous solution is cooled to room temperature, add deionized water (the amount is 5 times the volume of the homogeneous solution) to obtain a suspension;
[0041] (3) Centrifuge the suspension, wash with water and ethanol, and dry at 110° C. for 12 hours to obtain the zeolite-like imidazolate framework material. The performance test results of the zeolite-like imidazolate framework material prepared in this example are shown in Table 1. The X-ray diffraction pattern of the zeolite-like imidazolate framewor...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com