Method for preparing methylprednisolone
A technology of methylprednisolone and dichloromethane, applied in the field of chemical preparation, can solve the problems of high price, complicated process, cumbersome steps and the like, and achieve the effects of low cost, good selectivity and simple reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
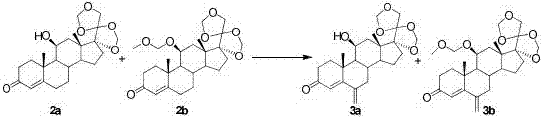
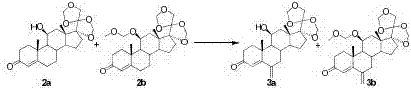
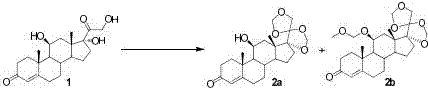
[0047] The first step, ketal protection reaction: dissolve hydrocortisone 1 (10g, 1W) in dichloromethane (200mL, 20V), add formaldehyde aqueous solution (100mL, 10V) under stirring, slowly add concentrated hydrochloric acid (100ml, 10V ), stirred at room temperature for 8 hours, TLC detected no raw material point, stopped the reaction, separated the organic phase, extracted the aqueous phase with dichloromethane, and combined the organic phases. The organic phase was washed successively with saturated aqueous sodium bicarbonate solution and saturated aqueous sodium chloride solution, and dried over anhydrous magnesium sulfate. The organic solvent was removed by rotary evaporation under reduced pressure to obtain a mixture of protected products 2a and 2b (11.02g, 110%), product 2a HPLC (240nm, 55%), and product 2b HPLC (240nm, 37%).
[0048] The second step, methylation reaction: Add sodium acetate (20g, 2W), dichloromethane (100mL, 10V) and methylal (200mL, 20V) into the react...
Embodiment 2
[0055] The first step, ketal protection reaction: dissolve hydrocortisone 1 (10g, 1W) in dichloromethane (100mL, 10V), add formaldehyde aqueous solution (46mL, 4.6V) under stirring, slowly add concentrated hydrochloric acid (42ml, 4.2V), stirred at 30°C for 10 hours, stopped the reaction, separated the organic phase, extracted the aqueous phase with dichloromethane, and combined the organic phases. The organic phase was washed successively with saturated aqueous sodium bicarbonate solution and saturated aqueous sodium chloride solution, and dried over anhydrous magnesium sulfate. The organic solvent was removed by rotary evaporation under reduced pressure to obtain a mixture of protected products 2a and 2b (10.4g, 104%), product 2a HPLC (240nm, 30%), product 2b HPLC (240nm, 40%).
[0056] Other steps are with embodiment 1.
Embodiment 3
[0058] The first step, ketal protection reaction: dissolve hydrocortisone 1 (10g, 1W) in dichloromethane (400mL, 40V), add formaldehyde aqueous solution (185mL, 18.5V) under stirring, slowly add concentrated hydrochloric acid (170ml, 17V), stirred at 20°C for 6 hours, stopped the reaction, separated the organic phase, extracted the aqueous phase with dichloromethane, and combined the organic phases. The organic phase was washed successively with saturated aqueous sodium bicarbonate solution and saturated aqueous sodium chloride solution, and dried over anhydrous magnesium sulfate. The organic solvent was removed by rotary evaporation under reduced pressure to obtain a mixture of protected products 2a and 2b (111g, 111%), product 2a HPLC (240nm, 50%), and product 2b HPLC (240nm, 43%).
[0059] Other steps are with embodiment 2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com