Enrofloxacin injection and production method thereof
A technology of enrofloxacin and its manufacturing method, which is applied in the field of injections, can solve the problems of unqualified foreign matter, user returns, waste, etc., and achieve the effects of product stability, product quality assurance, and prolongation of storage time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
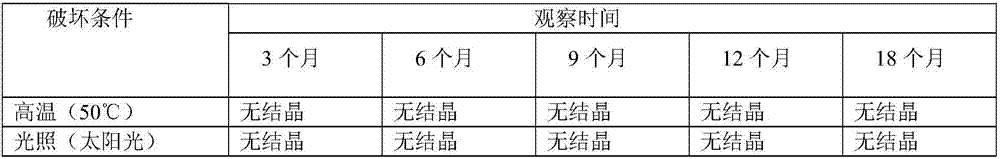
Examples
Embodiment 1
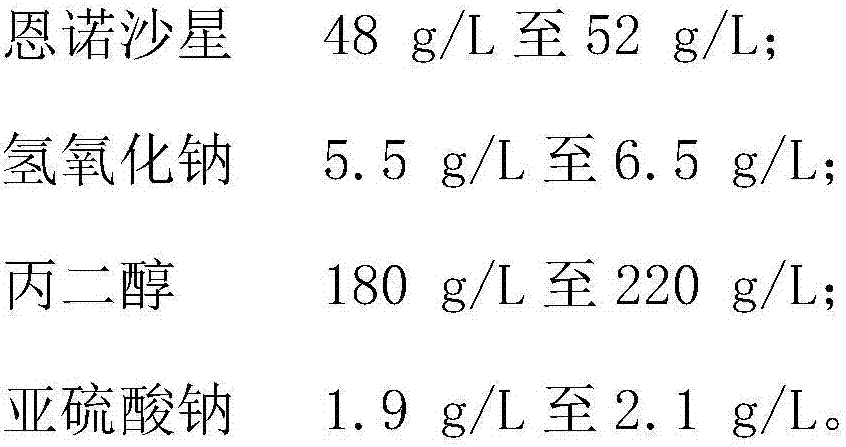
[0038] S1. Weigh 50g of enrofloxacin; 6g of sodium hydroxide; 200g of propylene glycol; 2g of anhydrous sodium sulfite.
[0039] S2. Add 200 ml of water for injection in the preparation container.
[0040] S3. Add the anhydrous sodium sulfite and sodium hydroxide in step S1 to the preparation container in step S2, and stir to fully dissolve the anhydrous sodium sulfite and sodium hydroxide.
[0041] S4. Heat the solution in the preparation container so that the temperature of the solution is kept at 30° C. to 40° C., add the enrofloxacin in step S1 under stirring, and stir until the enrofloxacin is completely dissolved.
[0042] S5. Add the propylene glycol in step S1 to the preparation container, and add water for injection to the second predetermined amount, so that the total volume of the solution is 995 ml.
[0043] S6. Adjust the pH value with hydrochloric acid or sodium hydroxide, the concentration of hydrochloric acid or sodium hydroxide is 0.1N, adjust the pH value at...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com