Non-oily doramectin injection as well as preparation method and application thereof
A doramectin and non-oily technology, which is applied in the field of non-oily doramectin injection and its preparation, can solve the problems of easy rancidity or discoloration of oil, high difficulty of filtration sterilization, and poor application effect, so as to promote dissolution , The preparation method is simple and efficient, and the use effect is good
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0036] A preparation method of the above-mentioned non-oily doramectin injection, comprising the following steps:
[0037] Take 0.1%-10% doramectin by weight percentage, and add it into a solvent mixed with glycerol formal and propylene glycol, wherein the mass ratio of the glycerol formal to the propylene glycol is (4-8): (2- 6);
[0038] The solvent and the doramectin are mixed, stirred evenly, subpackaged by blowing nitrogen, capped, sterilized, passed the light inspection and packaged to obtain the non-oily doramectin injection.
[0039] In addition, it is also necessary to provide a non-oily doramectin injection for preventing, treating or controlling parasites in animals.
[0040] The non-oily doramectin injection in the present invention can be used for deworming in vivo and in vitro of poultry, livestock and other animals. For example, the injection can be used for subcutaneous or intramuscular injection of animals for in vivo deworming, or the injection can be appli...
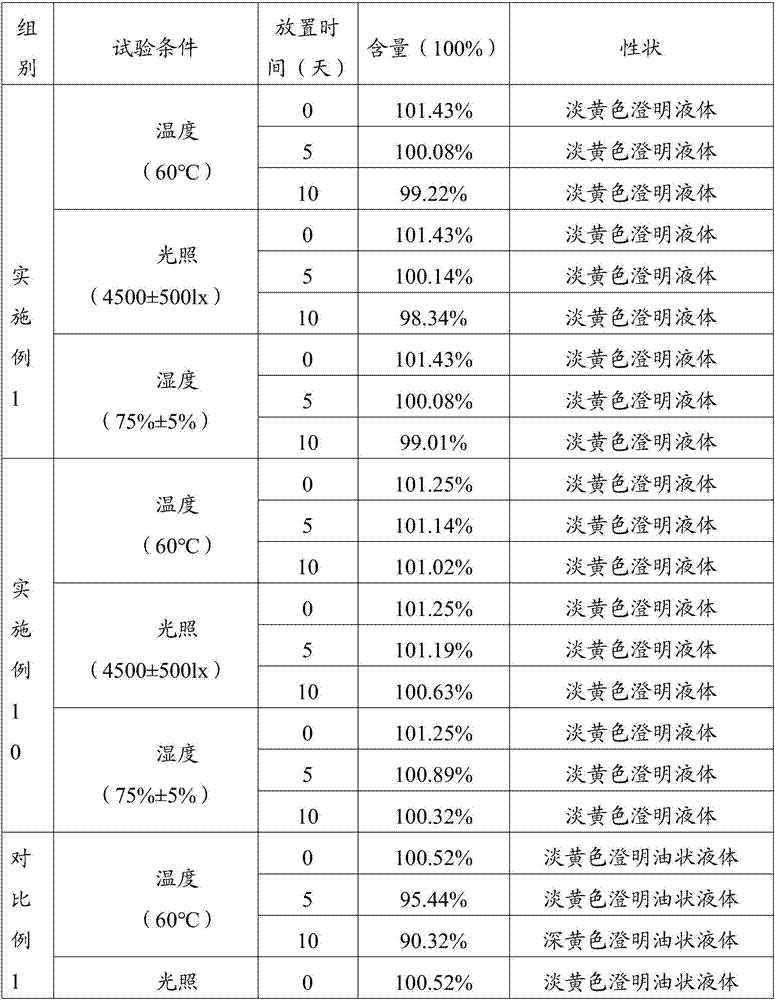
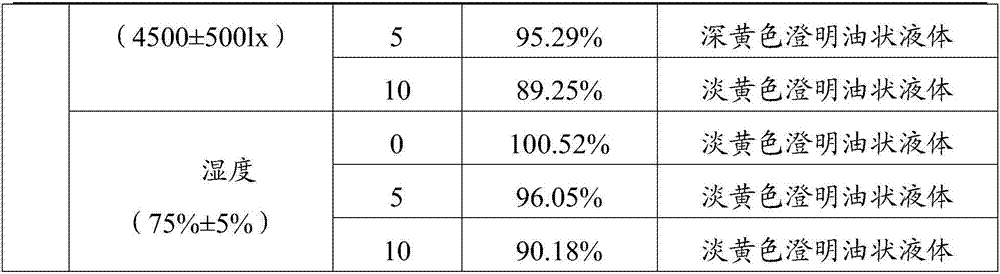
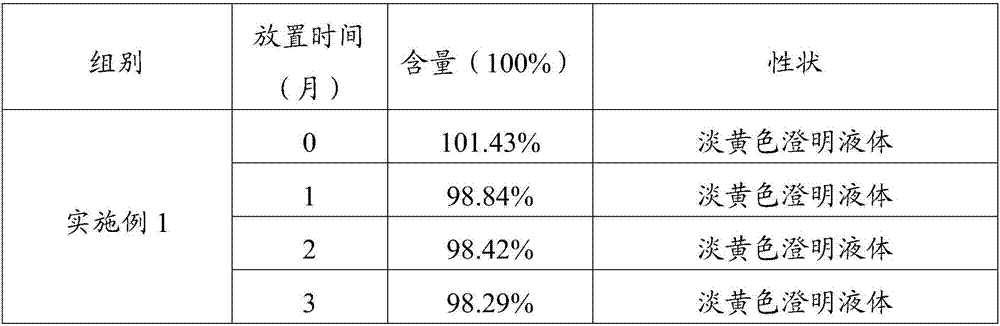
Embodiment 1
[0043] Weigh each raw material with the following weight percentage (take the total amount of 100ml as an example)
[0044] Doramectin 1%, glycerin formal 59.4% and propylene glycol 39.6%.
[0045] 1) Add glycerin formal and propylene glycol into the stirring tank, and mix well;
[0046] 2) Add the doramectin into the mixing tank in step 1), and stir for 15 minutes until it is completely dissolved;
[0047] 3) The solution obtained in step 2) is subpackaged by blowing nitrogen, capped, sterilized, passed the lamp inspection and packaged to obtain the non-oily doramectin injection 1.
Embodiment 2
[0049] Weigh each raw material with the following weight percentage (take the total amount of 100ml as an example)
[0050] Doramectin 1%, glycerin formal 39.7% and propylene glycol 59.3%.
[0051] The preparation method of this non-oily doramectin injection 2 is the same as that of Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com