Picromerite preparation method based on brine mixing method
A technology of strontium and brine mixing method, applied in the field of comprehensive utilization of salt lake brine, can solve the problems of inability to be reused, waste of land occupation, pollution in the salt lake area, etc. Simple process effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
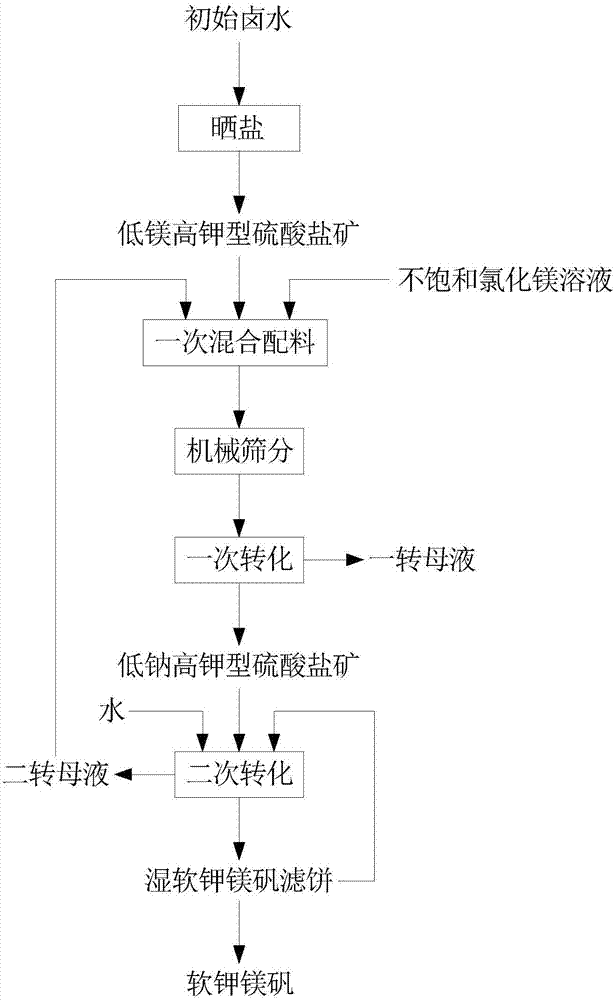
[0022] The invention provides a kind of preparation method based on the lenbeinite of mixing brine method, specifically refer to figure 1 , the preparation method comprises the following steps:
[0023] Step S1, drying the initial brine to obtain low-magnesium and high-potassium sulfate ore.
[0024] Specifically, the initial brine refers to the Na + The mass percentage is 3.0% ~ 5.0%, Mg 2+ The mass percentage is 2.0% ~ 4.5%, K + The mass percentage is 0.5% ~ 1.0%, Cl - The mass percentage of SO is 13.0%~16.0%, SO 4 2- The mass percentage is 3.0%~6.0% salt lake brine, or waste brine or intermediate brine etc. produced in the utilization process of salt lake brine; in the embodiment of the present invention, Keke Salt Lake is preferably produced after producing sodium chloride Discarded brine was used as the initial brine.
[0025] Na + The mass percentage is 18.0% ~ 20.0%, Mg 2+ The mass percentage is 1.5% ~ 4.0%, K + The mass percentage is 5.0% ~ 8.0%, Cl - The ma...
Embodiment 1
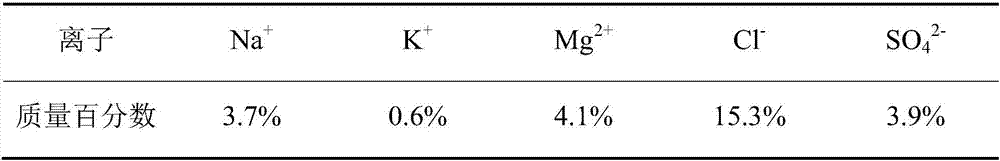
[0039] In this example, the initial brine used comes from the waste brine produced after the production of sodium chloride in Keke Salt Lake, and its main ion composition is shown in Table 1.
[0040] Table 1 The main ion composition of the initial brine
[0041]
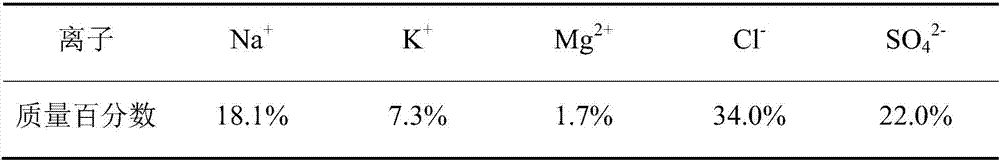
[0042] After step S1, the main ion composition of the obtained low-magnesium and high-potassium sulfate ore is shown in Table 2.
[0043] Table 2 Main ion composition of low-magnesium and high-potassium sulfate ores
[0044]
[0045] In step S2, the mass ratio of the low-magnesium and high-potassium sulfate ore, the unsaturated magnesium chloride solution, and the second transfer mother liquor is controlled to be 2:1:1, and after mixing the ingredients once, stir for about 15 minutes to remove chlorine by mechanical sieving Sodium chloride; then carry out a transformation at room temperature for 30 minutes, and separate the solid and liquid to obtain a solid low-sodium high-potassium sulfate ore and a mother ...
Embodiment 2
[0056] In the description of Embodiment 2, the similarities with Embodiment 1 will not be repeated here, and only the differences with Embodiment 1 will be described. The difference between Example 2 and Example 1 is that in step S1, the main ion composition of the initial brine is shown in Table 5.
[0057] The main ion composition of table 5 initial brine
[0058]
[0059] After step S1, the main ion composition of the obtained low-magnesium and high-potassium sulfate ore is shown in Table 6.
[0060] Table 6 The main ion composition of low-magnesium and high-potassium sulfate ore
[0061]
[0062] In step S2, control the mass ratio of low-magnesium and high-potassium sulfate ore, unsaturated magnesium chloride solution, and the second transfer mother liquor to 2.5:1:0.7, and then perform mechanical sieving to remove chlorine after mixing the ingredients for about 10 minutes. Sodium chloride; then carry out a transformation at room temperature for 25 minutes, and sep...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com