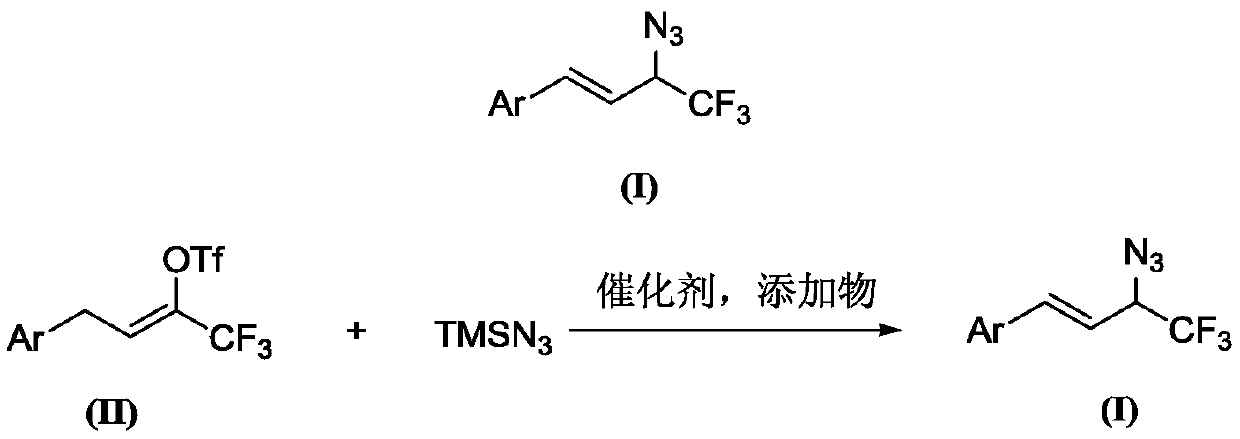
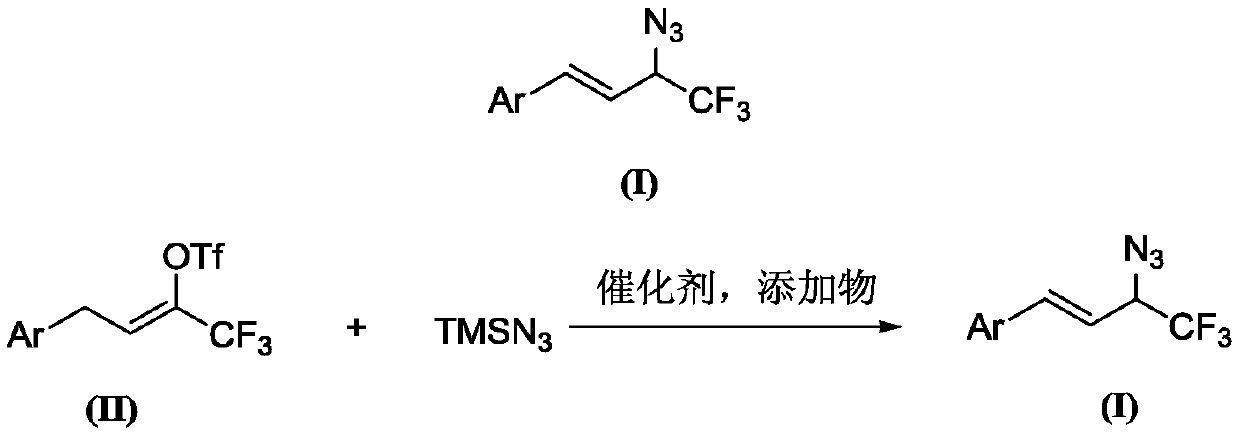
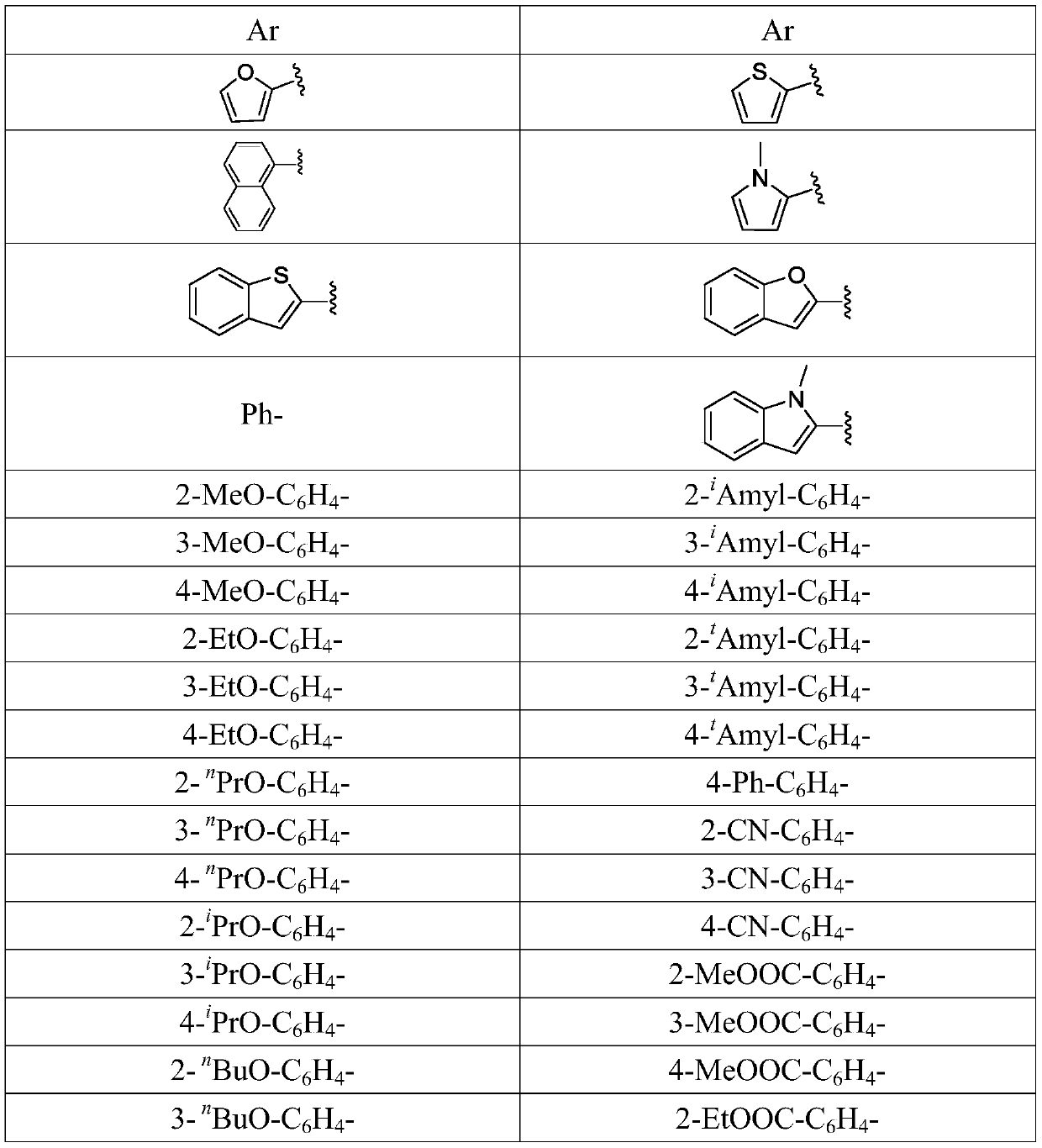
A kind of preparation method of 1-aryl-3-azido-4,4,4-trifluoro-1-butene compound
A compound, azido-based technology, applied in the field of compound preparation, can solve problems such as limited application, expensive, unstable, etc., and achieve low-cost effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Preparation of 1-phenyl-3-azido-4,4,4-trifluoro-1-butene (Compound 1)
[0056] At room temperature, add 167 mg (0.5 mmol) of 4-phenyl-2-trifluoromethanesulfonate-1,1,1-trifluoro-2-butene and anhydrous solvent to a 25mL Schlenk bottle protected by argon. Ethylene glycol dimethyl ether 5mL, 4-phenyl-2-trifluoromethanesulfonate-1,1,1-trifluoro-2-butene 1.2 times the molar amount of potassium bicarbonate 60mg (0.6mmol), 4 -Phenyl-2-trifluoromethanesulfonate-1,1,1-trifluoro-2-butene 0.14mL (1.0mmol), 4-phenyl-2- 0.76 mL (10 mg / mL, 0.075 mmol) of triethylamine catalyst with 0.15 times molar amount of trifluoromethanesulfonate-1,1,1-trifluoro-2-butene was placed at 65°C for 12 hours of reaction. Extracted with ethyl acetate (3×10 mL), the combined organic phase was washed with saturated brine (2×10 mL) and dried over anhydrous magnesium sulfate. The target compound was obtained by column chromatography. The filler was silica gel and the eluent was petroleum. The ether separatio...
Embodiment 2
[0058] Preparation of 1-(4-methylphenyl)-3-azido-4,4,4-trifluoro-1-butene (Compound 2)
[0059] Except that 4-phenyl-2-trifluoromethanesulfonate-1,1,1-trifluoro-2-butene in Example 1 was replaced with the same molar amount of 4-(4-methylphenyl) Except for -2-trifluoromethanesulfonate-1,1,1-trifluoro-2-butene, the procedure was carried out in the same manner as in Example 1, and the target compound was obtained in an isolated yield of 69%.
Embodiment 3
[0061] Preparation of 1-(4-isopropylphenyl)-3-azido-4,4,4-trifluoro-1-butene (Compound 3)
[0062] Except that the 4-phenyl-2-trifluoromethanesulfonate-1,1,1-trifluoro-2-butene in Example 1 was replaced with the same molar amount of 4-(4-isopropylphenyl )-2-Trifluoromethanesulfonyl-1,1,1-trifluoro-2-butene was carried out in the same manner as in Example 1, and the target compound was obtained with an isolated yield of 77%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com