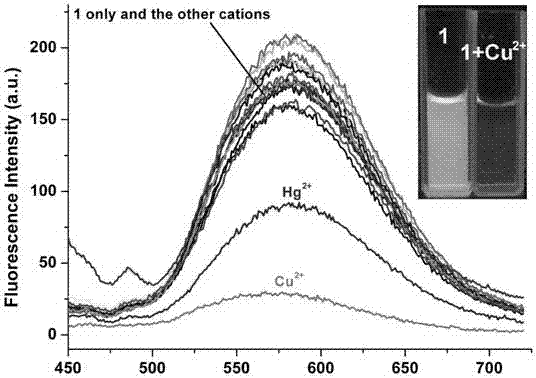
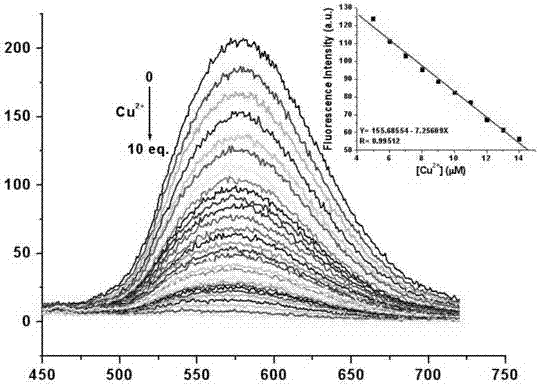
Fluorescent probe based on pyrrole-cumarin dihydrazone derivatives as well as preparation method and application of fluorescent probe
A fluorescent probe, coumarin technology, applied in fluorescence/phosphorescence, chemical instruments and methods, luminescent materials, etc., can solve problems such as nervous system disorders, and achieve the effects of high sensitivity, strong selectivity, and simple synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Synthesis of pyrrole-coumarin dihydrazone derivatives
[0026]
[0027] Dissolve 195mg of 3,5-dimethylpyrrole-2-carbaldehyde in 10mL of absolute ethanol, then add 218mg of 4-hydroxy-3-acetylcoumarin hydrazone, and drop in 2 drops of glacial acetic acid, reflux and stir for 3h under normal pressure After cooling to room temperature, a large amount of solids were precipitated, filtered under reduced pressure, and the filter residue was washed with absolute ethanol to obtain a yellow solid, which was the target product, and the yield of the target product was 68%.
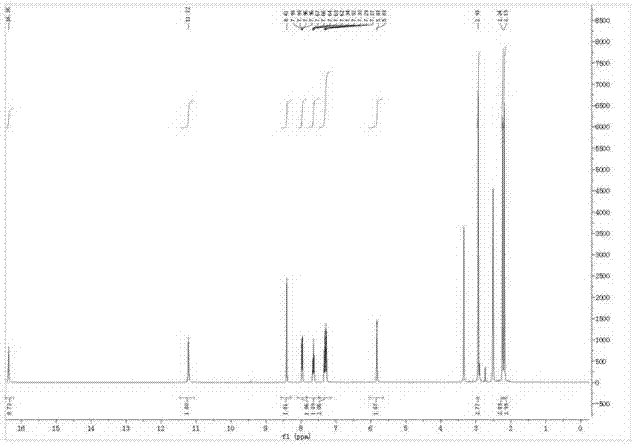
[0028] Adopt nuclear magnetic resonance instrument to carry out nuclear magnetic resonance analysis to the pyrrole-coumarin dihydrazone derivative that makes, the result is as follows:
[0029] 1 H NMR (400MHz, DMSO-d 6 ), δ (ppm): 16.36 (s, 1H, OH), 11.22 (s, 1H, NH), 8.41 (s, 1H, CH=N), 7.96-7.98 (d, 1H, Aryl-H), 7.62 -7.66(t,1H,Aryl-H),7.27-7.34(q,1H,Aryl-H),5.83(s,1H,CH),2.93(s,3H,CH 3 ),2.19(s,3H,CH...
Embodiment 2
[0032] Dissolve 390mg of 3,5-dimethylpyrrole-2-carbaldehyde in 20mL of 95% ethanol, then add 436mg of 4-hydroxy-3-acetylcoumarin hydrazone, drop in 2 drops of glacial acetic acid, reflux under normal pressure for 3h, cool After reaching room temperature, a large amount of solid was precipitated, filtered under reduced pressure, and the filter residue was washed with 95% ethanol to obtain a yellow solid, which was the target product, and the yield of the target product was 72%.
Embodiment 3
[0034] Dissolve 195mg of 3,5-dimethylpyrrole-2-carbaldehyde in 10mL of 75% ethanol, then add 218mg of 4-hydroxy-3-acetylcoumarin hydrazone, drop in 2 drops of glacial acetic acid, reflux under normal pressure for 3h, cool After reaching room temperature, a large amount of solid was precipitated, filtered under reduced pressure, and the filter residue was washed with 75% ethanol to obtain a yellow solid, which was the target product, and the yield of the target product was 78%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com