Long wavelength type H2S fluorescence probe, compounding method and application thereof
A technology of fluorescent probe and synthesis method, which is applied to long-wavelength H2S fluorescent probe and its synthesis and application fields, can solve the problems of long response time, short emission wavelength, complex synthesis route, etc., and achieves high selectivity and good sensitivity , the effect of easy separation and purification process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Embodiment 1: The specific synthetic steps of compound 2 are as follows:
[0035]
[0036]Compound 1,4-diethyl-1,2,3,4-tetrahydro-7-hydroxyquinoxaline-6-al (468mg, 2mmol), ethyl acetoacetate (260mg, 2mmol) and piperidine 0.1mL Dissolve in ethanol and reflux for 4 hours, then pour the solution into hydrochloric acid (4mol / L, 10mL), stir at 60°C for 30 minutes, mix the solution with water, extract with ethyl acetate (3×80mL), and wash with anhydrous sulfuric acid After drying over sodium, the crude product was purified by silica gel column chromatography using EA:PE=1:5 (v / v) as the eluent, and 420.5 mg of compound 2 was isolated with a yield of 70%. 1 H NMR (400MHz, CDCl 3 )δ8.38(s,1H),6.48(s,1H),6.39(s,1H),3.59-3.52(m,2H),3.42(q,J=7.1Hz,2H),3.32(q,J =7.1Hz, 2H), 3.26-3.20(m, 2H), 2.67(s, 3H), 1.20(dt, J=15.9, 7.1Hz, 6H).
Embodiment 2
[0037] Embodiment 2: The specific synthetic steps of compound 1 are as follows:
[0038]
[0039] Compound 2 (300.4mg, 1mmol), p-hydroxybenzaldehyde (134.2mg, 1.1mmol), and piperidine (0.1mmol) were dissolved in toluene and refluxed for 4h, then hydrochloric acid (4mol / L, 15mL) was added and stirred at 40°C For 50 minutes, add water to the solution, then extract with ethyl acetate (3×100mL), dry the organic layer over anhydrous sodium sulfate, and purify the crude product by silica gel column chromatography using EA:PE=1:2 (v / v) As eluent, 179.8 mg of compound 1 was isolated with a yield of 44%. 1 H NMR (400MHz, DMSO-d 6 )δ10.01(s,1H),8.48(s,1H),7.82(d,J=15.7Hz,1H),7.56(d,J=15.7Hz,1H),7.51(d,J=8.2Hz, 2H), 6.76-6.83(m, 3H), 6.51(s, 1H), 3.51(t, J=5.1Hz, 2H), 3.45(q, J=7.1Hz, 2H), 3.27(d, J=7.1 Hz, 2H), 3.15 (t, J=5.1Hz, 2H), 1.08 (dt, J=13.2, 5.1Hz, 6H).
Embodiment 3
[0040] Embodiment 3: The specific synthetic steps of fluorescent probe L are as follows:
[0041]
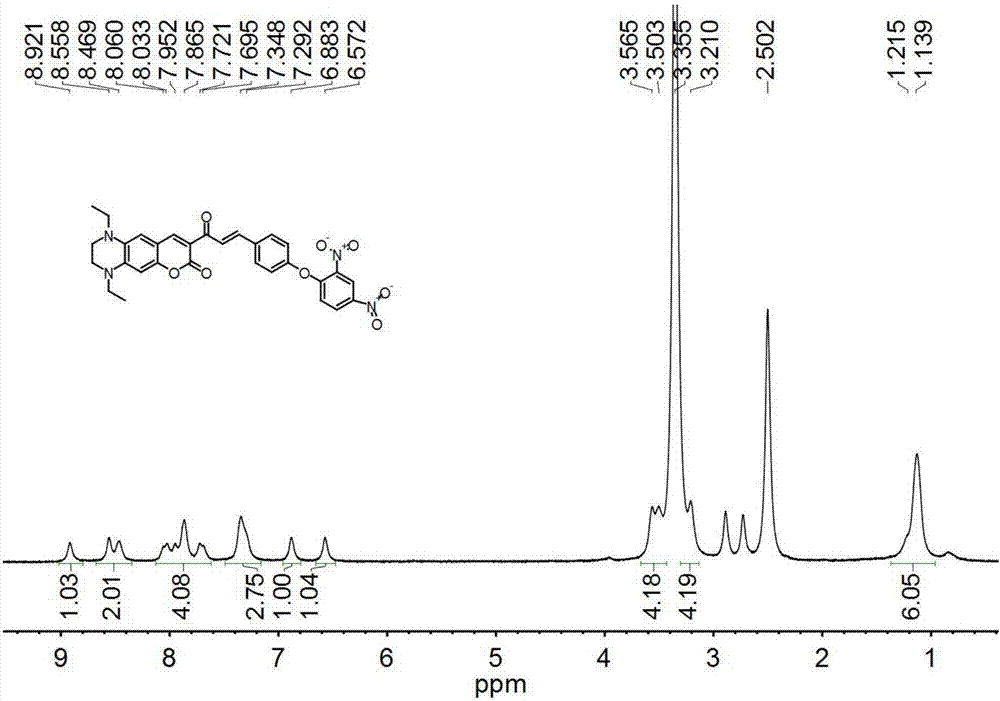
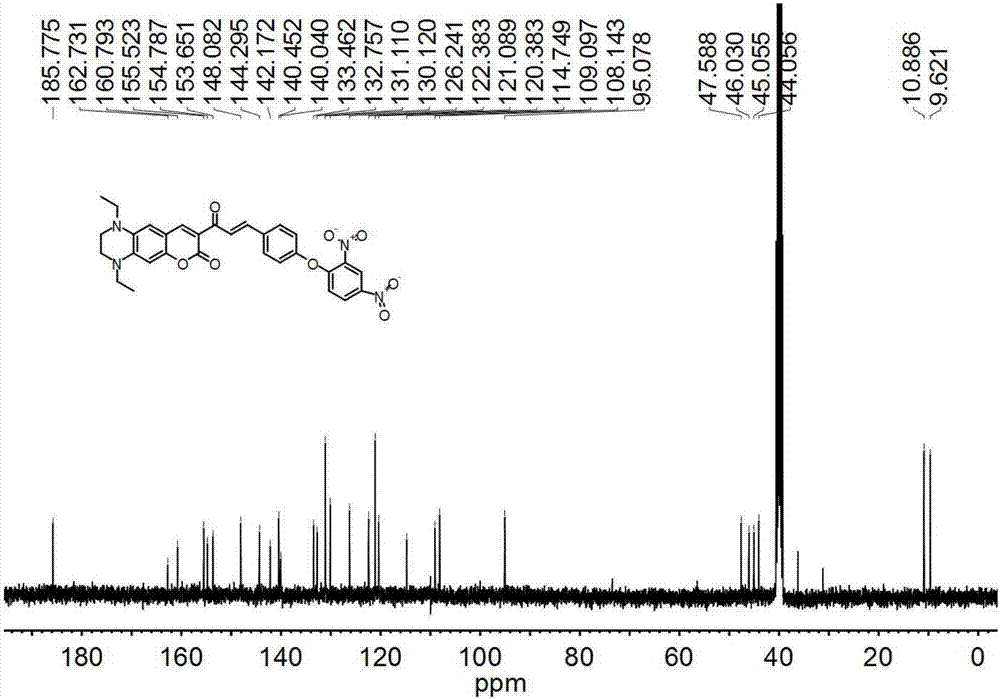
[0042] Compound 1 (404.5mg, 0.5mmol), 2,4-dinitrofluorobenzene (186mg, 1.0mmol), potassium carbonate (187mg, 1.35mmol), dissolved in DMF (5mL) and stirred, reacted at room temperature for 3h . After the reaction was completed, it was quenched with water, extracted with ethyl acetate (3×100mL), dried over anhydrous sodium sulfate, and the solvent was spinned out to obtain the crude product, which was purified by silica gel column chromatography using PE:PE=1:10 (v / v) was used as eluent to obtain 285.3 mg of probe L, yield 50%. 1 H NMR (400MHz, DMSO-d 6 )δ8.92(s,1H),8.55(s,1H),8.46(s,1H),7.69-8.06(m,4H),7.29-7.34(m,3H),6.88(s,1H),6.57 (s,1H),3.21-3.56(m,8H),1.13-1.21(m,6H). 13 C NMR (101MHz, DMSO-d 6 )δ185.77,162.73,160.79,155.52,154.79,153.65,148.08,144.30,142.17,140.45,140.04,133.46,132.76,131.11,130.12,126.24,122.38,121.09,120.38,114.75,109.10,108.14,95.08,47.59,46.03 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com