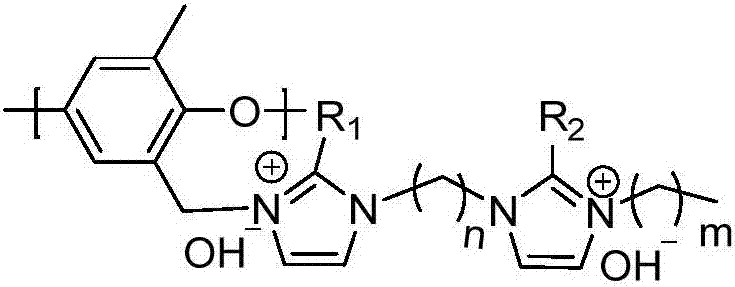
Biimidazole cation alkaline anion exchange membrane based on polyphenylene ether and preparation method thereof
A basic anion and biimidazolium cation technology, which is applied in the field of biimidazolium cation basic anion exchange membrane and its preparation, can solve the problems of low mechanical strength and difficult membrane electrodes, and achieve simple preparation process, excellent alkali resistance, and preparation The effect of simple and safe process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
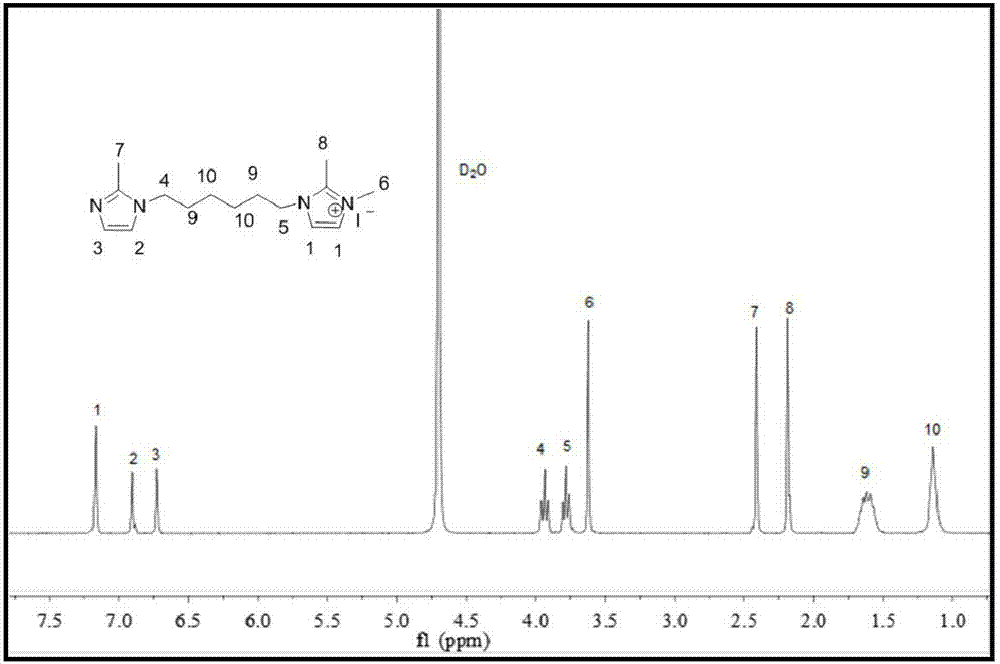
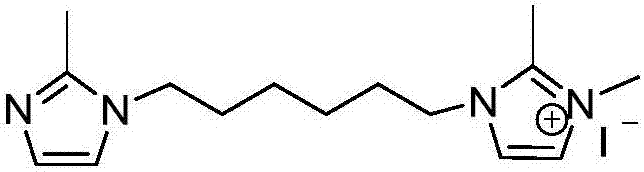
[0026] Dissolve 3.00g (36.50mmol) 2-methylimidazole in 25ml acetonitrile, add 4.45g (18.25mmol) 1,6-dibromohexane and 4.1g (73.00mmol) KOH, nitrogen protection, room temperature (25°C, The same below), reacted for 24 hours, dissolved the crude product in water, extracted the crude product with dichloromethane, repeated three times, and dried the product to obtain a white solid product;
[0027] 1.00g (4.06mmol) of the white solid product prepared above and 0.58g (4.06mmol) of methyl iodide were dissolved in 25ml of ethyl acetate, reacted at room temperature for 4 hours, separated and purified to obtain the functional monomer containing the biimidazole structure, the molecule The structure is:
[0028]
[0029] The preparation of other functional monomers containing bis-imidazole structure can also refer to this preparation method.
Embodiment 2
[0031] 0.4g (2.86mmol) bromination degree is 25% BrPPO and 0.74g (2.86mol) prepared in Example 1 Dissolve in 25ml of N-methylpyrrolidone, react at 30°C for 6 hours, place the mixed solution in a polytetrafluoroethylene template, and dry at 70°C for 2 hours to prepare a halogen-type anion exchange membrane;
[0032] Place the obtained halogen-type anion exchange membrane in 1M KOH solution and soak it at 60°C for 24 hours. After the halogen anion is completely exchanged into OH-, remove the residual KOH with deionized water to obtain the OH-type anion-exchange membrane.
[0033] The OH-type anion exchange membrane obtained in this example has a water absorption rate of 55.56%, a swelling degree of 21.36%, an ion exchange capacity of 1.25 mmol / g, and an ion conductivity of 11.57 mS cm at room temperature. -1 , the ion conductivity at 90°C is 32.40mS·cm -1 .
[0034] The membrane was immersed in 1M KOH solution for 400 hours, and its conductivity was measured again. The ion co...
Embodiment 3
[0036] 0.4g (2.71mmol) of BrPPO with a bromination degree of 35% and 0.67g (2.71mmol) Dissolve in 25ml of N-methylpyrrolidone, react at 50°C for 4 hours, place the mixed solution in a polytetrafluoroethylene template, and dry at 70°C for 2 hours to prepare a halogen-type anion exchange membrane;
[0037] Place the obtained halogen-type anion exchange membrane in 1M KOH solution and soak it at 60°C for 24 hours. After the halogen anion is completely exchanged into OH-, remove the residual KOH with deionized water to obtain the OH-type anion-exchange membrane.
[0038] The OH-type anion exchange membrane obtained in this example has a water absorption rate of 58.14%, a swelling degree of 21.58%, an ion exchange capacity of 1.31 mmol / g, and an ion conductivity of 14.23 mS cm at room temperature. -1 , the ion conductivity at 90°C is 35.02mS·cm -1 .
[0039] The membrane was immersed in 1M KOH solution for 400 hours, and its conductivity was measured again. The ion conductivity ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| water absorption | aaaaa | aaaaa |
| water absorption | aaaaa | aaaaa |
| water absorption | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com