Prophylactic/therapeutic agent for virus infections which comprises ala compound
A technology for infectious diseases and therapeutic agents, applied in the field of prevention and/or therapeutic agents for viral infectious diseases, can solve problems such as teratogenic effects, administration to women who cannot conceive, and inability to directly remove viruses, and achieves fewer side effects and survival rate. Improve and avoid the effect of severe aggravation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
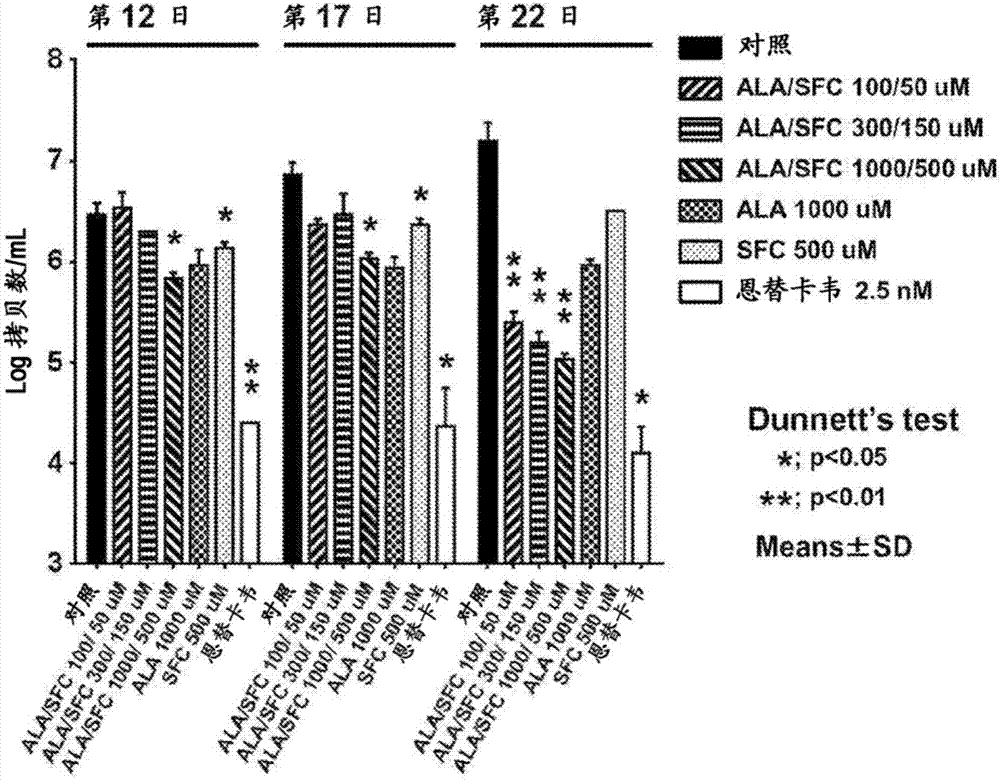
[0101] (tested cells)
[0102] As a test sample, more than 70% of the mouse hepatocytes were replaced with normal human hepatocytes, so that the liver showed a metabolism close to that of the human liver. In order to infect human hepatitis B and C viruses, it was used in pharmacokinetic studies. PXB hepatocytes prepared from "PXB mouse (registered trademark)" (PhoenixBio Co., Ltd.), which is used as an in vivo prediction model of hepatitis virus infection in humans, are added to a medium such as 5-ALA, and The resulting changes in viral load were investigated.
[0103] (Preparation of medium for HBV infection)
[0104] In dHCGM medium containing 4% polyethylene glycol (DMEM+10%FBS, 44mM NaHCO 3 , 15 μg / mL of L-proline, 0.25 μg / mL of insulin, 50 μM of dexamethasone, 5 ng / mL of EGF, 0.1 mM of Asc-2P, 2% of DMSO), suspend each well as 2× 10 6 Individual hepatitis B virus (HBV) (genotype C) was prepared into 250 μL / well HBV infection medium. It should be noted that the sequen...
Embodiment 2
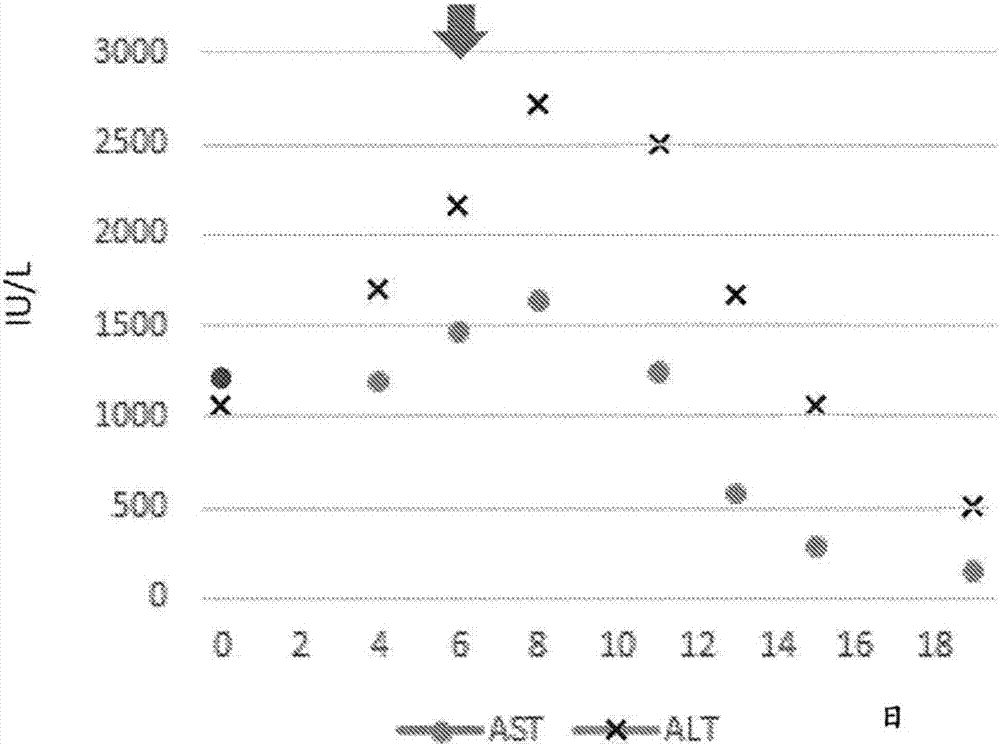
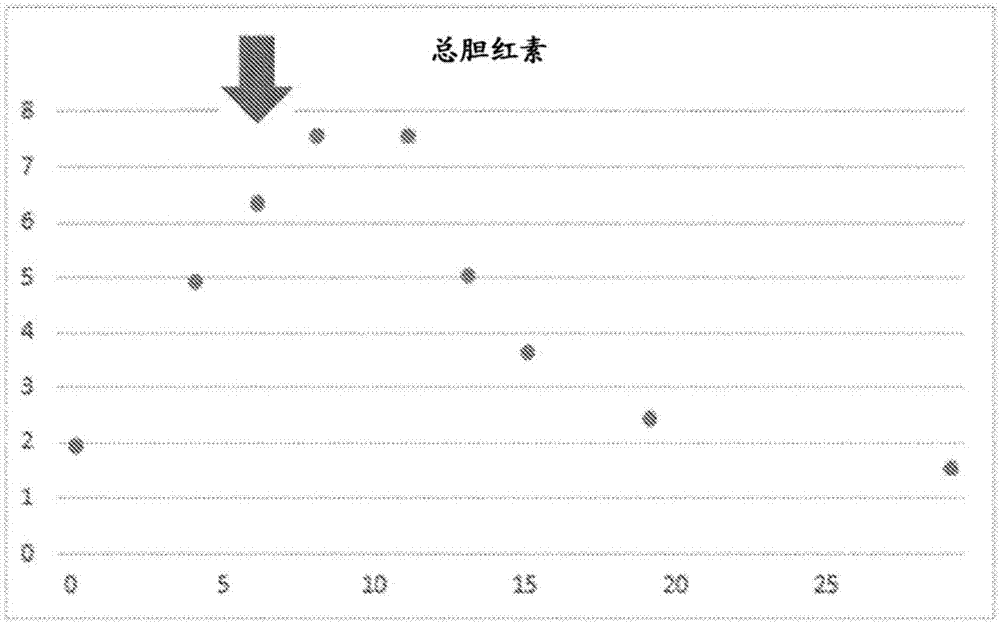
[0117] For a 54-year-old male patient with hepatitis B, on the 6th day of admission (taking the day of admission as day 0), oral administration of ALA phosphate containing 220 mg and sodium ferrous citrate (SFC) (ALA-SFC) 63 mg was started. ) capsules (one capsule per day), this day is regarded as the 0th day of administration. After the start of the administration, the value of total bilirubin including aspartate aminotransferase (AST) which is considered to increase in the value of blood as the destruction of liver cells progresses was measured. ), alanine aminotransferase (alanine aminotransferase: ALT), and conjugated bilirubin that is thought to leak from bile into the blood due to liver damage, and non-conjugated bilirubin that is thought to increase in value due to destruction of red blood cells type bilirubin etc. show the result in figure 2 and image 3 .
[0118] (result)
[0119] Depend on figure 2 It shows that until the 2nd day of ALA-SFC administration (t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com