A kind of preparation method of metformin hydrochloride sustained release tablet
A technology for metformin hydrochloride and sustained-release tablets, which can be applied to pharmaceutical formulas, medical preparations containing no active ingredients, medical preparations containing active ingredients, etc., and can solve problems such as uneven drug release, large proportion, and unfavorable swallowing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
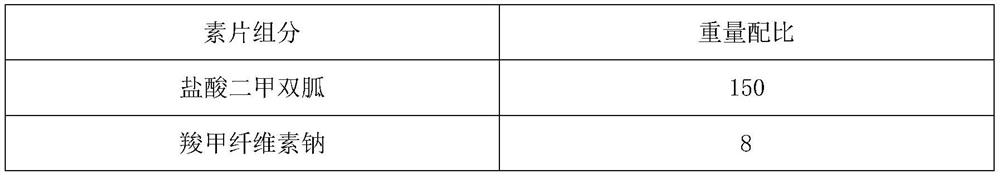
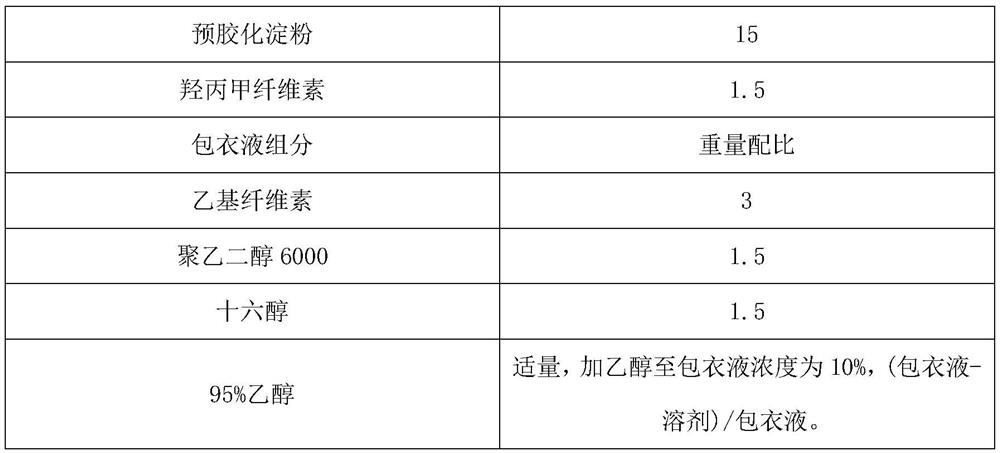
[0028] Embodiment 1: a kind of metformin hydrochloride slow-release tablet preparation method comprises the following steps:
[0029] S1: Weigh metformin hydrochloride, carmellose sodium, pregelatinized starch, and hypromellose according to the ratio, and set aside;
[0030] S2: Take the hypromellose weighed in S1 to prepare a binder solution, wherein the solvent is purified water, and the mass fraction of the binder is 4%;
[0031] S3: Weigh the ethyl cellulose and other components according to the ratio, and set aside. The other components are polyethylene glycol 6000 and cetyl alcohol. Under stirring, the weighed ethyl cellulose, polyethylene glycol Add alcohol 6000 and cetyl alcohol to 95% ethanol solution, continue to stir until completely dissolved, soak overnight, pass through a 125-mesh sieve, and set aside.
[0032] S4: Metformin hydrochloride, carmellose sodium and pregelatinized starch are uniformly pulverized through a 85-mesh sieve, stirred and mixed for 30 minut...
Embodiment 2
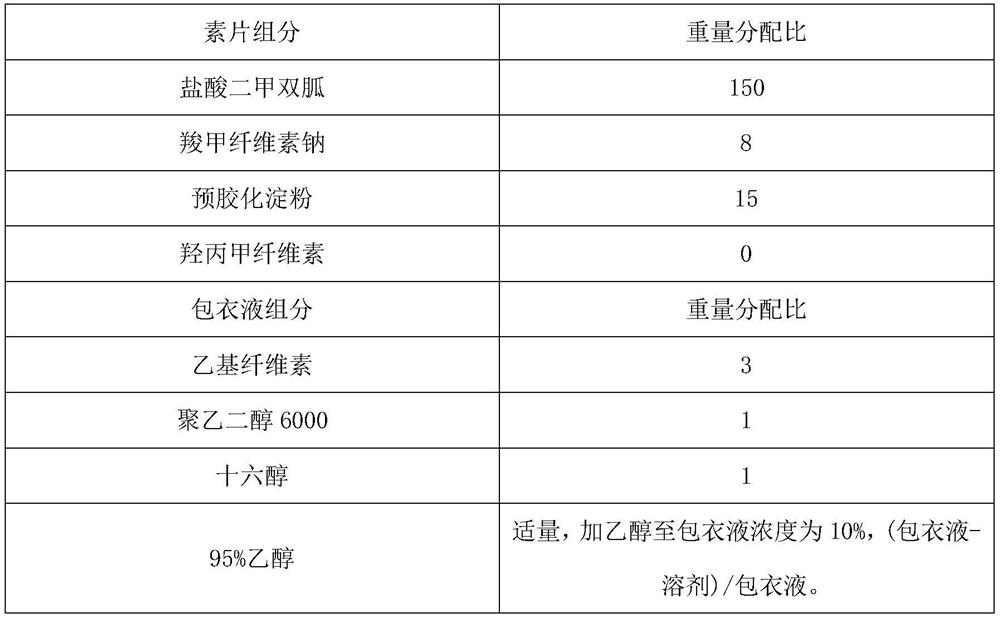
[0039] Embodiment 2: a kind of metformin hydrochloride slow-release tablet preparation method comprises the following steps:
[0040] S1: Weigh metformin hydrochloride, carmellose sodium, pregelatinized starch, and hypromellose according to the ratio, and set aside;
[0041] S2: Take the hypromellose weighed in S1 to prepare a binder solution, wherein the solvent is purified water, and the mass fraction of the binder is 4%;
[0042] S3: Weigh the ethyl cellulose and other components according to the ratio, and set aside. The other components are polyethylene glycol 6000 and cetyl alcohol. Under stirring, the weighed ethyl cellulose, polyethylene glycol Add alcohol 6000 and cetyl alcohol to 95% ethanol solution, continue to stir until completely dissolved, soak overnight, pass through a 125-mesh sieve, and set aside;
[0043] S4: Metformin hydrochloride, carmellose sodium and pregelatinized starch are uniformly pulverized through a 85-mesh sieve, stirred and mixed for 30 minutes...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com