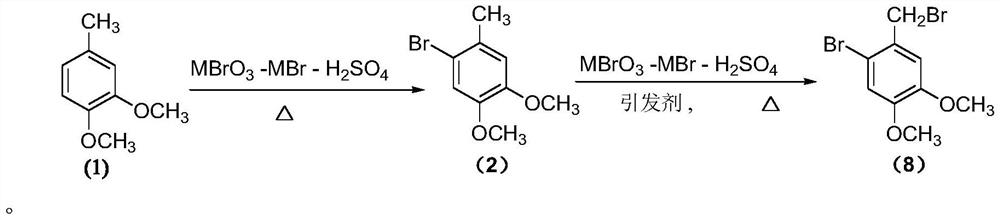
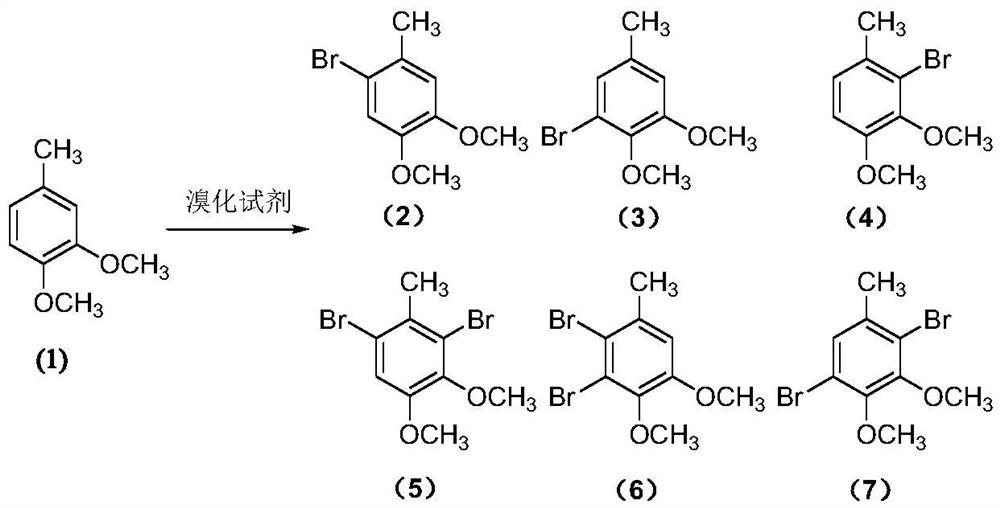
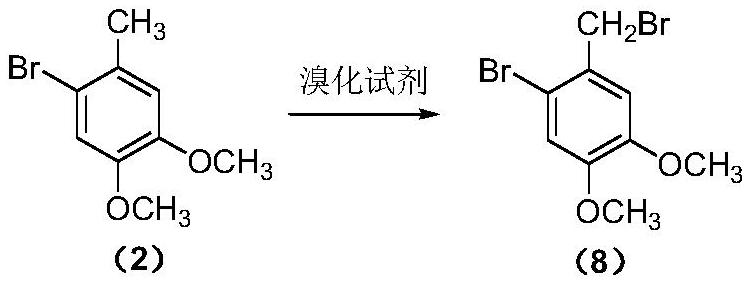
A kind of preparation method of pinaverium bromide intermediate 2-bromo-4,5-dimethoxybenzyl bromide
A technology of dimethoxybenzyl bromide and pinaverium bromide, which is applied in the field of preparation of 2-bromo-4,5-dimethoxybenzyl bromide, can solve the problems of high production cost, large amount required, and large environmental pollution, etc. problems, to avoid the formation of multi-substituted products, promote free radical substitution reactions, and avoid by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] 3,4-Dimethoxytoluene (7.6g, 50mmol), sodium bromate (6.0g, 40mmol), sodium bromide (8.2g, 80mmol) and carbon tetrachloride (18mL) were added to a stirring, In the reaction flask of the reflux condenser, thermometer and tail gas absorption device, heat to reflux, slowly add 1 / 2 volume of sulfuric acid (diluted 60mmol concentrated sulfuric acid with 4mL water, add it within about 1.5h), track with gas chromatography, After the substrate fully participates in the reaction, quickly add 1 / 3 volume of the initiator solution (0.25g ABVN is dissolved in 7mL carbon tetrachloride to obtain), after the system appears violent reflux, continue to slowly add the remaining sulfuric acid and the initiator solution (about Add within 4.5h), continue to react until the product no longer forms or when by-products increase significantly (about 1.5h), cool down to room temperature, filter, and the filtrate is washed with 10ml sodium bicarbonate solution (5%), let stand to separate the liquid,...
Embodiment 2
[0055] 3,4-Dimethoxytoluene (7.6g, 50mmol), sodium bromate (6.0g, 40mmol), sodium bromide (8.2g, 80mmol) and carbon tetrachloride (18mL) were added to a stirring, In the reaction flask of the reflux condenser, thermometer and tail gas absorption device, heat to reflux, slowly add 1 / 2 volume of sulfuric acid (diluted 60mmol concentrated sulfuric acid with 4mL water, add it within about 1.5h), track with gas chromatography, After the substrate fully participates in the reaction, quickly add 1 / 3 volume of the initiator solution (0.16g AIBN is dissolved in 7mL carbon tetrachloride to obtain), after the system appears violent reflux, continue to slowly add the remaining sulfuric acid and the initiator solution (about Add within 4.5h), continue to react until the product no longer forms or when the by-products increase significantly (about 1.5h), cool down to room temperature, filter, and the filtrate is washed with 10ml sodium bicarbonate solution (5%), separated, and the organic ph...
Embodiment 3
[0057] 3,4-Dimethoxytoluene (7.6g, 50mmol), sodium bromate (6.0g, 40mmol), sodium bromide (8.2g, 80mmol) and carbon tetrachloride (38mL), were added to a stirring, Into the reaction flask of the reflux condenser, thermometer and tail gas absorption device, heat to reflux, slowly add 1 / 2 volume of sulfuric acid (diluted with 60mmol of concentrated sulfuric acid with 4mL of water, add it within about 1h), track with gas chromatography, the bottom After the substance completely participates in the reaction, quickly add 1 / 3 volume of initiator solution (0.25g of azobisisoheptanonitrile dissolved in 7mL of carbon tetrachloride to obtain), after the system appears violent reflux, continue to slowly add the remaining sulfuric acid and trigger (about 4.5h added), continue to react until the product is no longer formed or by-products increase significantly (about 1.5h), down to room temperature, filtered, the filtrate was washed with 10ml sodium bicarbonate solution (5%), separated liq...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com