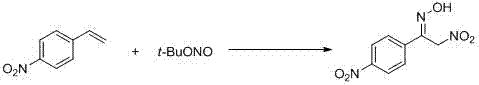
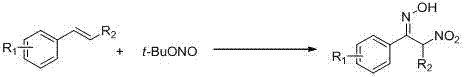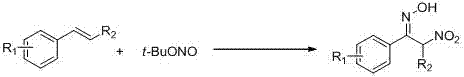Preparation method of alpha-nitro ketoxime derivative
A technology for nitroketoxime and styrene derivatives, which is applied in oxime preparation, organic chemistry and other directions, can solve the problems of environmental pollution, expensive transition metals, and difficulty in meeting the demand for oxime compounds, and achieves environmental friendliness and shortens the reaction time. The effect of high reaction time and yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-7
[0022] Example 1-7 Optimization of reaction conditions
[0023] Using styrene as the raw material, tert-butyl nitrite as the reagent, and water as the solvent (2mL), the effects of various conditions on the reaction effect were explored, and representative examples 1-7 were selected. The results are as follows Table 1 shows:
[0024]
[0025] Table I:
[0026] Example
Auxiliary (mol%)
Time(h)
T ( o C)
Yield (%)
1
—
0.5
25
12
2
—
12
25
28
3
—
24
25
62
4
—
36
25
87
5
—
48
25
84
6
PEG400 (20%)
0.5
25
91
7
PEG600 (20%)
0.5
25
89
[0027] The basic reaction conditions are as follows: 0.4 mmol styrene, 0.8 mmol tert-butyl nitrite, 2 mL water, and air.
[0028] Taking Example 1 as an example, the specific operation is as follows: Under air conditions, add styrene (0.4mmol), tert-butyl nitrite (0.8mmol) and solvent water (2mL) to a 10mL round-bottom flask reactor. Stir at room temperature for 30 minutes. After the reaction, the reaction solution was washed with saturated brine and extracted with ...
Embodiment 7
[0033]
[0034] Under air conditions, add p-chlorostyrene (0.4mmol), tert-butyl nitrite (0.8mmol), PEG400 (0.08mmol) and solvent water (2mL) into a 10mL round-bottom flask reactor, and stir for 30min at room temperature After the reaction, the reaction solution was washed with saturated brine and extracted with ethyl acetate (3x10 mL). After the organic phase was dried over anhydrous magnesium sulfate, the organic solvent was removed by rotary evaporation, and the residue was separated by silica gel column chromatography to obtain the target product (Yield 83%).
Embodiment 8
[0036]
[0037] Under air conditions, add p-methoxystyrene (0.4mmol), tert-butyl nitrite (0.8mmol), PEG400 (0.08mmol) and solvent water (2mL) to a 10mL round-bottom flask reactor, at room temperature Stir for 30 min. After the reaction is complete, the reaction solution is washed with saturated brine and extracted with ethyl acetate (3x10 mL). After drying the organic phase over anhydrous magnesium sulfate, the organic solvent is removed by rotary evaporation, and the residue is separated by silica gel column chromatography. The target product (yield 87%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com