Cadmium organic framework compound and preparation method thereof
A technology of organic frameworks and complexes, applied in the field of transition metal complexes, can solve problems such as no reports, and achieve the effects of simple preparation method, low cost and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Embodiment 1. Complex of the present invention [Cd (L) (H 2 O) 2 ] n preparation method
[0021] Weigh 0.05mmol H 2 L with 0.1mmolCd(NO 3 ) 2 4H 2 O was placed in a 15ml teflon tube, and 8ml H was added 2 O and 2ml DMF, stirred at room temperature for 30 minutes. Then the polytetrafluoroethylene tube was sealed in a stainless steel reaction kettle, the temperature was controlled at 120°C and heated for 72 hours, and it was naturally lowered to room temperature to obtain a colorless diamond-shaped crystal, which was washed three times with distilled water and dried in vacuum. The yield of the obtained cadmium complex was was 76%. Infrared spectrum (wave number, cm -1 ): 3446(m), 3134(m), 2309(w), 1837(w), 1764(w), 1588(s), 1534(s), 1442(m), 1410(m), 1362(s ),1335(s),1282(s),1233(m),1216(m),1142(s),1075(m),1000(w),980(s),914(m),886(m ), 814(s), 671(m), 653(m), 567(w).
Embodiment 2
[0022] Embodiment 2. Complexes of the present invention [Cd(L)(H 2 O) 2 ] n crystal structure of
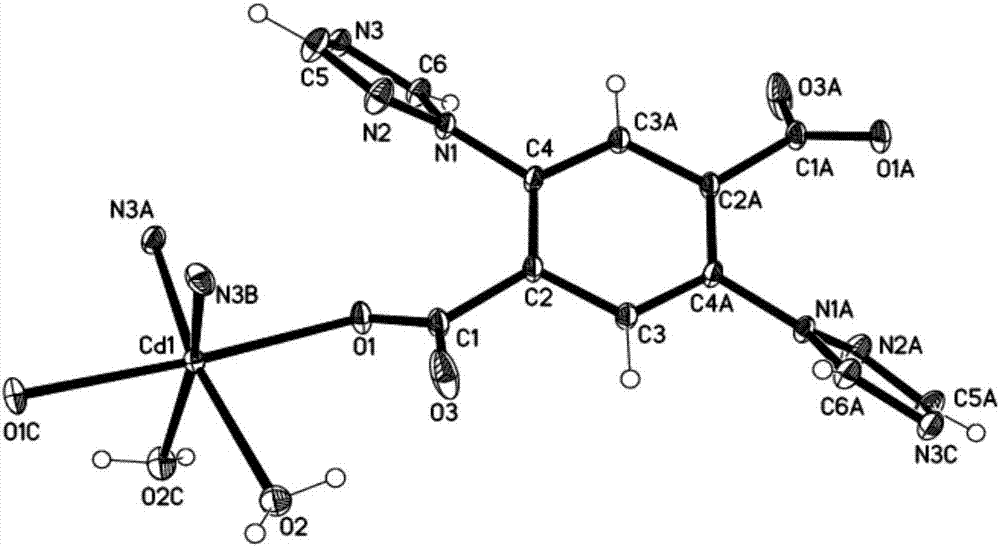
[0023] Select a single crystal of a suitable size under a microscope, use X-ray diffraction, use Bruker Smart Apex II detector to monochromatize Mo-Kα rays through a graphite monochromator, scan mode ω, and collect diffraction data at room temperature. All diffraction data were subjected to semi-empirical absorption correction using the SADABS program after SAINT reduction. The crystal structure was solved by SHELXL-97 direct method. The detailed crystal determination data are shown in Table 1; the crystal structure is as figure 1 As shown, each Cd(II) adopts a hexacoordinated mode, L 2- Four coordination atoms are provided to coordinate with four central ions Cd(II), thereby forming a three-dimensional network structure.
[0024] Crystallographic data of the complexes in Table 1
[0025]
[0026]
Embodiment 3
[0027] Embodiment 3. Complexes of the present invention [Cd(L)(H 2 O) 2 ] n Infrared Spectrum
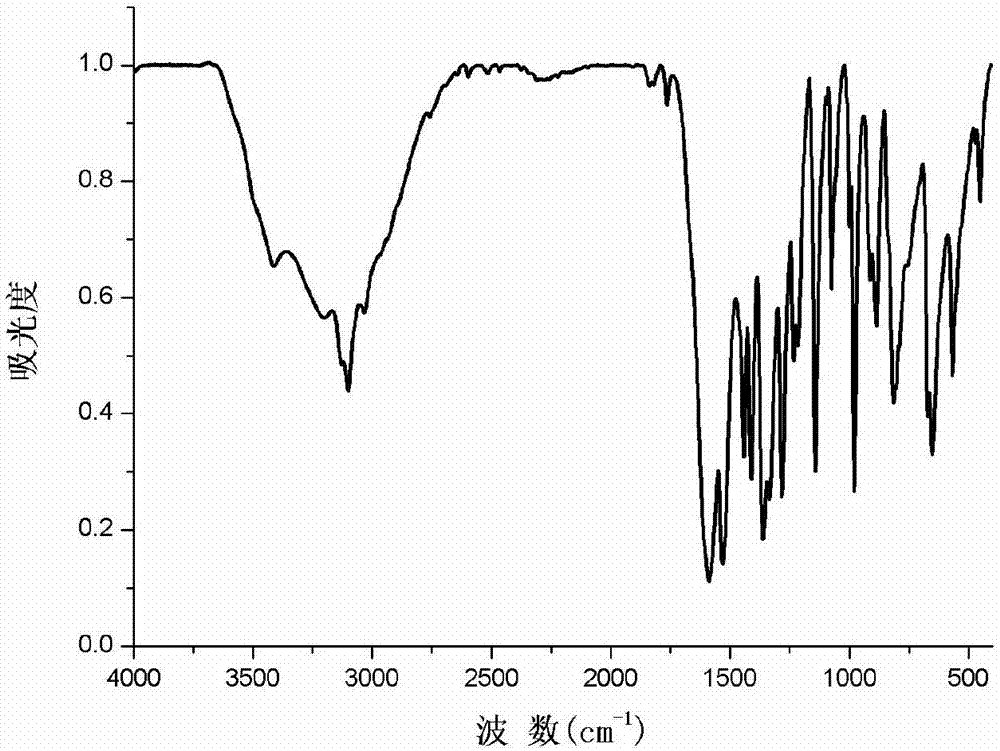
[0028] Take a small amount of [Cd(L)(H 2 O) 2 ] n Solid powder, pressed with KBr, recorded in infrared spectrometer at 4000-400cm -1 absorb, see figure 2 . Complex characteristic absorption peak (cm -1 ) and the corresponding vibration modes are: the complex at 3134cm -1 The broadband absorption of corresponds to the O–H stretching vibration of water molecules. Complex at 1700cm -1 The characteristic band at is very weak compared with the ligand, indicating that the carboxyl group reacts with Cd(II) ion to completely deprotonate.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com