Covalent organic frameworks-supported cobalt catalyst and preparation and application thereof
A technology of covalent organic frameworks and cobalt catalysts, which is applied in organic compound/hydride/coordination complex catalysts, organic chemistry, physical/chemical process catalysts, etc., can solve the problems of limiting the application of catalysts, and achieve scalable, Promote the effect of smooth progress and high added value of products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
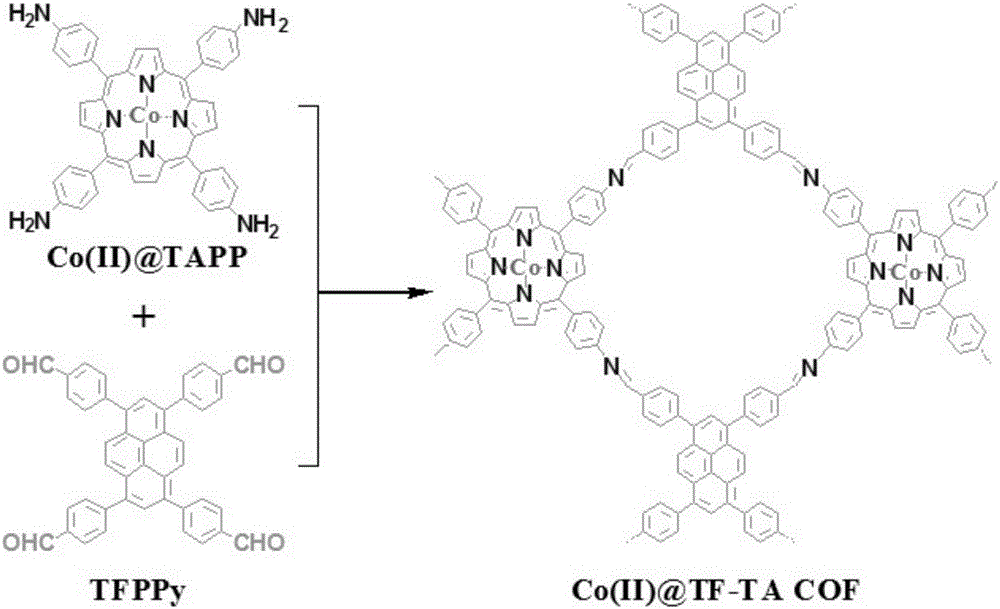
[0023] Step 1: Synthesis of Co(II)@TF-TA COF: Add Co(II)TAPP (36.6mg, 0.05mmol) and TFPPy (30.9mg, 0.05mmol) powders to a 10mL ampoule, and then add the mixed solvent 1,4-dioxane / mesitylene / N,N'-dimethylacetamide / 3mmol / L acetic acid solution (0.7mL / 0.7mL / 0.5mL / 0.1mL), sonicate the ampoule after addition The powder is fully dispersed and dissolved in the solvent. Put the ampoule into liquid nitrogen to freeze the solvent into a solid, and seal the mouth of the ampoule under vacuum (~20Pa). Afterwards, the ampoule was placed in an oven at 140° C. for a static reaction for 3 days. The obtained solid powder was washed with acetone and filtered until the solvent was colorless, and dried at 100°C for 12 hours to obtain a brown solid powder Co(II)@TF-TACOF with a yield of 85%.
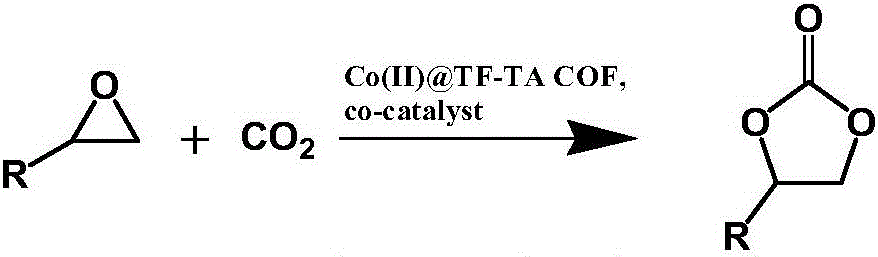
[0024] The second step: Co(II)@TF-TA COF is used as a heterogeneous catalyst to catalyze the reaction of carbon dioxide and epichlorohydrin to generate cyclocarbonate compounds. Under normal temperature an...
Embodiment 2-5
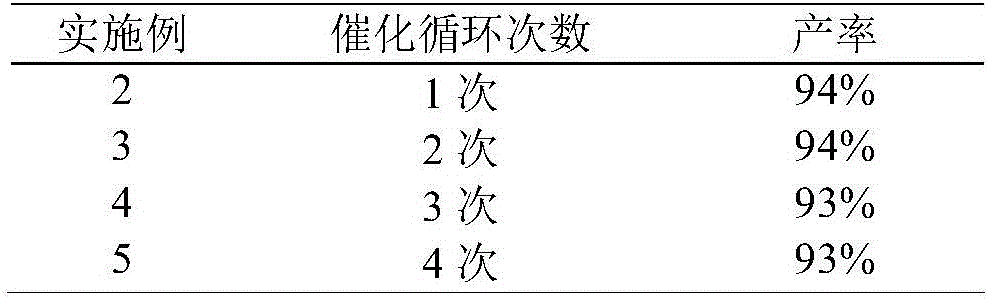
[0026]The Co(II)@TF-TA COF obtained in Example 1 was used as a heterogeneous catalyst to catalyze the reaction of carbon dioxide and epichlorohydrin to form a cyclocarbonate compound. The catalytic performance did not decrease significantly after being recycled for 4 times. According to Table 1 Described, other preparation, reaction, test conditions, with embodiment 1.
[0027] Table 1
[0028]
Embodiment 6-8
[0030] Under the catalysis of Co(II)@TF-TA COF obtained in Example 1, the reaction of carbon dioxide and epichlorohydrin was catalyzed to generate cyclocarbonate compounds. Different cocatalysts showed high yields for the reaction, according to Described in table 2, other preparation, reaction, test conditions, with embodiment 1.
[0031] Table 2
[0032]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com