A kind of visible light catalytic method for synthesizing pyrrol-4-one derivatives
A technology of visible light and derivatives, applied in chemical instruments and methods, chemical/physical processes, physical/chemical process catalysts, etc., can solve the problems of high equipment requirements, high cost, unfriendly environment, etc. Concise, low-dosage effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] A method for visible light catalytic synthesis of pyrrol-4-one, comprising the steps of:
[0056] 0.2 mmol cis ethyl 3-phenyl-3-(anilino)acrylate, 3.0 mg of photocatalyst 1 and 14.7 mg of zinc trifluoromethanesulfonate (Zn(OTf) 2 ) was added to 3mL of 1,4-dioxane to obtain solution A, and the concentration of photocatalyst 1 in the solution A was 1.3×10 -3 mol / L, the concentration of zinc trifluoromethanesulfonate is 1.3×10 -2 mol / L; In the air environment at room temperature, the solution A was irradiated with visible light for 1 h to react, and after the reaction was completed, rotary evaporation and column chromatography were carried out to obtain pyrrol-4-one.
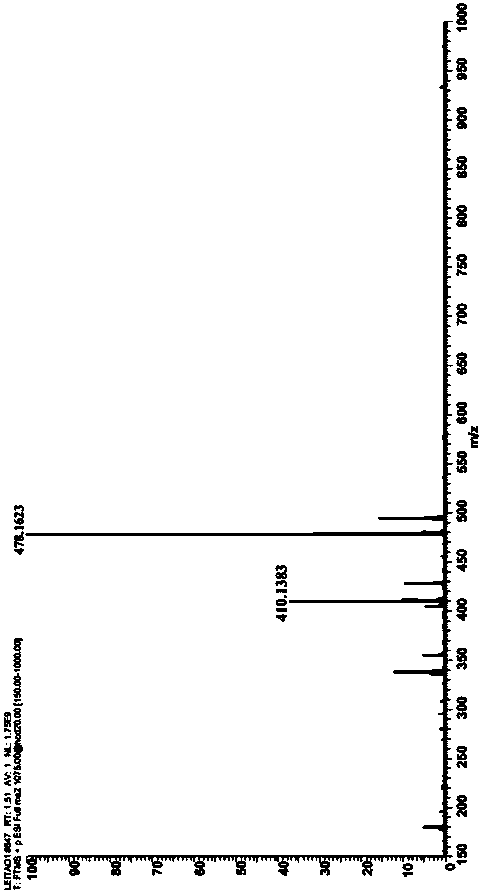
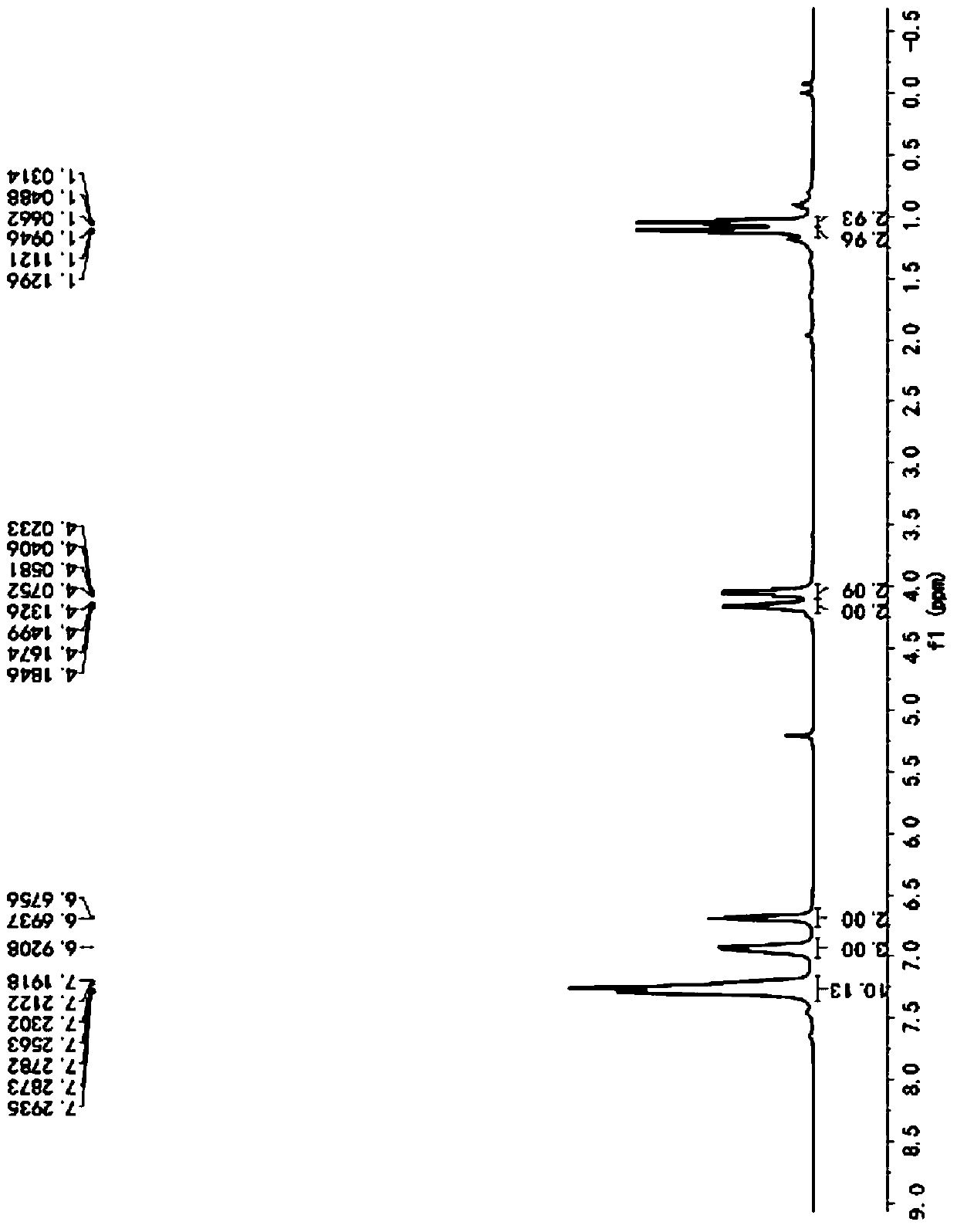
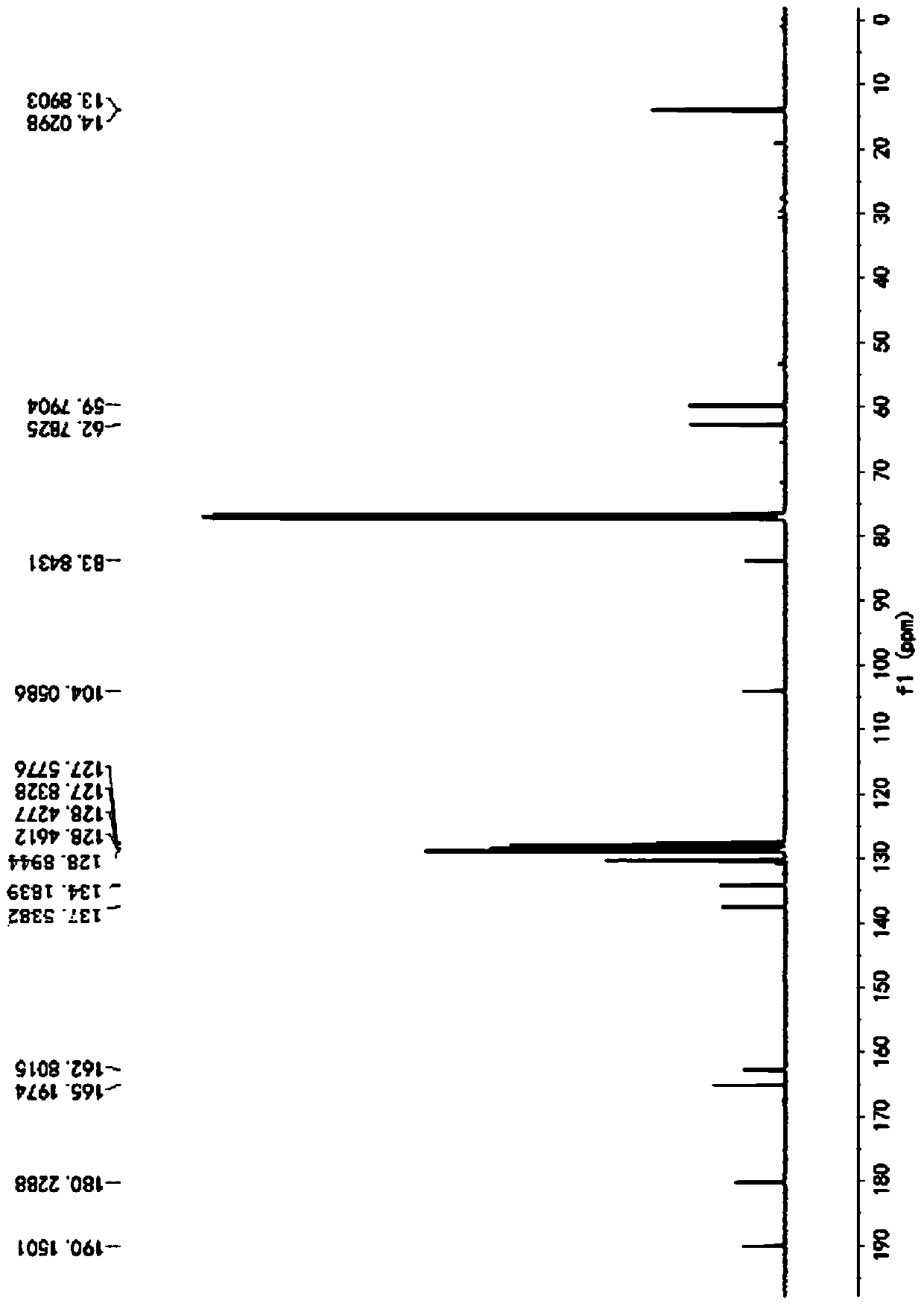
[0057] Described pyrrole-4-ketone is through the nuclear magnetic hydrogen spectrogram ( figure 1 )﹑carbon spectrum ( figure 2 ) and mass spectrum ( image 3 ) was determined to be ethyl 3-oxa-2,5-diphenyl-1-phenyl-2,3-dihydro-1H-pyrrole-2,4-dicarboxylate, and the conversion rate of the raw material was...
Embodiment 2 to 34
[0063] Except for the contents shown in Table 1, other pyrrol-4-ones were synthesized in the same manner as in Example 1.
[0064] The results of determination of the pyrrol-4-one by proton nuclear magnetic spectrum, carbon spectrogram and mass spectrogram are shown in Table 1.
[0065] Each parameter of table 1 synthetic pyrrol-4-ketone, concrete name and productive rate of pyrrol-4-ketone
[0066]
[0067]
[0068]
[0069]
[0070]
[0071]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com