A method for screening diabetic nephropathy drugs in vitro
A technology for diabetic nephropathy and in vitro screening, applied in the field of screening diabetic nephropathy drugs, can solve the problems of high cost, unstable model, complicated operation, etc., and achieve the effect of simple and easy operation, avoiding ethical problems and good integration.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
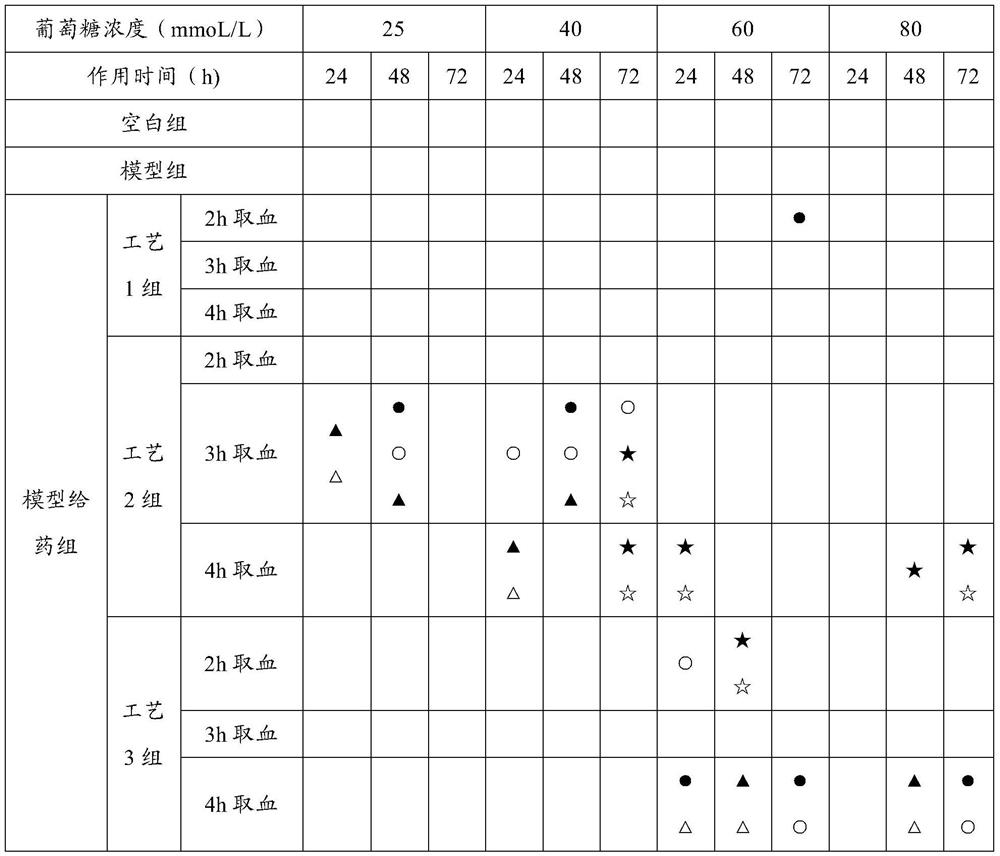
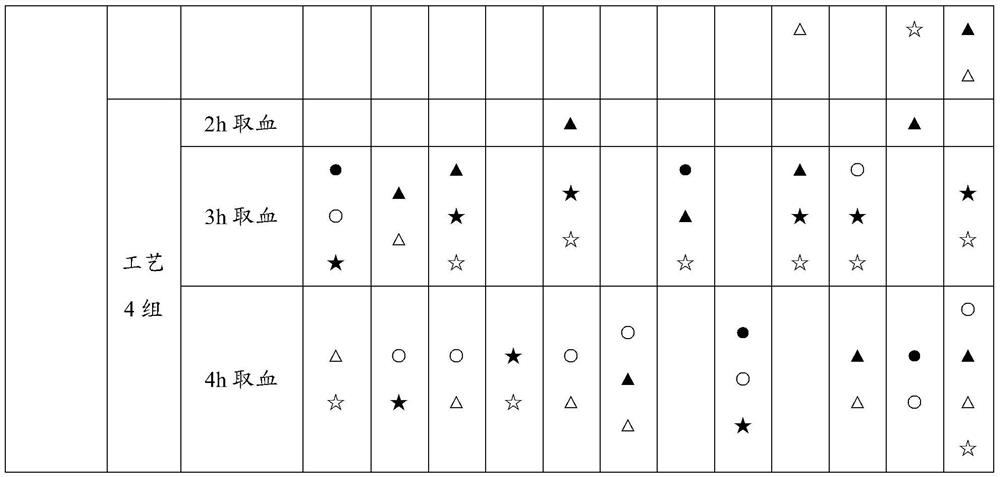
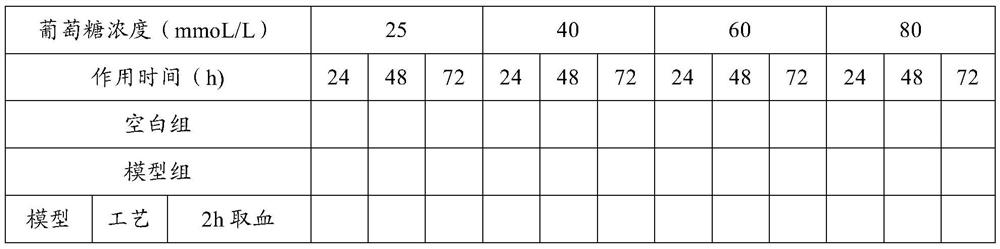
[0049] Example 1 Screening experiment on the preparation process of astragalus gorgon rhubarb traditional Chinese medicine composition
[0050] The purpose of the experiment: to screen the optimal preparation process of the traditional Chinese medicine composition through the method of serum pharmacology, combined with the in vitro drug efficacy screening method of the present invention, so as to lay the foundation for subsequent research and development.
[0051] 1. Experimental materials
[0052] (1) Experimental animals
[0053] 30 SPF grade SD rats, 180-220g, were purchased from Beijing Huafukang Biotechnology Co., Ltd., license number: SCXK (Beijing) 2014-0004, and were bred in Beijing Yizhuang International Biomedical Technology Co., Ltd., license number : SYXK (Beijing) 2015-0010.
[0054] (2) Experimental cells
[0055] Human renal tubular epithelial cells HKC were purchased from the Cell Resource Center of the Institute of Basic Medical Sciences, Chinese Academy of...
Embodiment 2
[0100] Embodiment 2 rhubarb preparation technology screening experiment
[0101] The results of Example 1 show that the drug effect of process 4 is better than that of process 3. The difference between process 4 and process 3 is only that the extraction solvent of rhubarb is different. In process 3, rhubarb is alcohol extraction, and in process 4, rhubarb is water extraction. In this experiment The glomerular mesangial cells and renal tubular epithelial cells most closely related to the onset of diabetic nephropathy were selected to test whether the water-extracted sample of rhubarb was more effective than the alcohol-extracted sample of rhubarb.
[0102] 1. Experimental materials
[0103] (1) Experimental animals
[0104] Animals 18 SPF grade SD rats, 180-220g, were purchased from Beijing Huafukang Biotechnology Co., Ltd., license number: SCXK (Beijing) 2014-0004, and were raised in Beijing Yizhuang International Biomedical Technology Co., Ltd., license No.: SYXK (Beijing) ...
Embodiment 3
[0149] Example 3 Astragalus gorgon rhubarb traditional Chinese medicine composition process 3 and process 4 animal experiments
[0150] The results of Examples 1 and 2 show that Process 4 is superior to Process 3, and even better than Processes 1 and 2 in terms of inhibiting proliferation of renal intrinsic cells and increasing glucose intake under high glucose conditions. In order to further confirm the reliability of the proposed screening method, the in vivo animal experiment was conducted again through the type 1 SD rat DN model to test the conclusion of the in vitro cell screening test.
[0151] 1. Experimental materials
[0152] (1) Experimental animals
[0153] 57 SPF grade SD rats, 180-220g, were purchased from Beijing Huafukang Biotechnology Co., Ltd., license number: SCXK (Beijing) 2014-0004, and were bred in Beijing Yizhuang International Biomedical Technology Co., Ltd., license number : SYXK (Beijing) 2015-0010.
[0154] (2) Experimental drugs
[0155] Prescrip...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com