A fixed-bed dehydrogenation process for low-carbon alkanes
A low-carbon alkanes, fixed-bed technology, applied in the fields of butane dehydrogenation to butene and propane dehydrogenation to propylene, to achieve the effect of reducing the amount of B acid, low equipment investment, and improved anti-carbon deposition performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
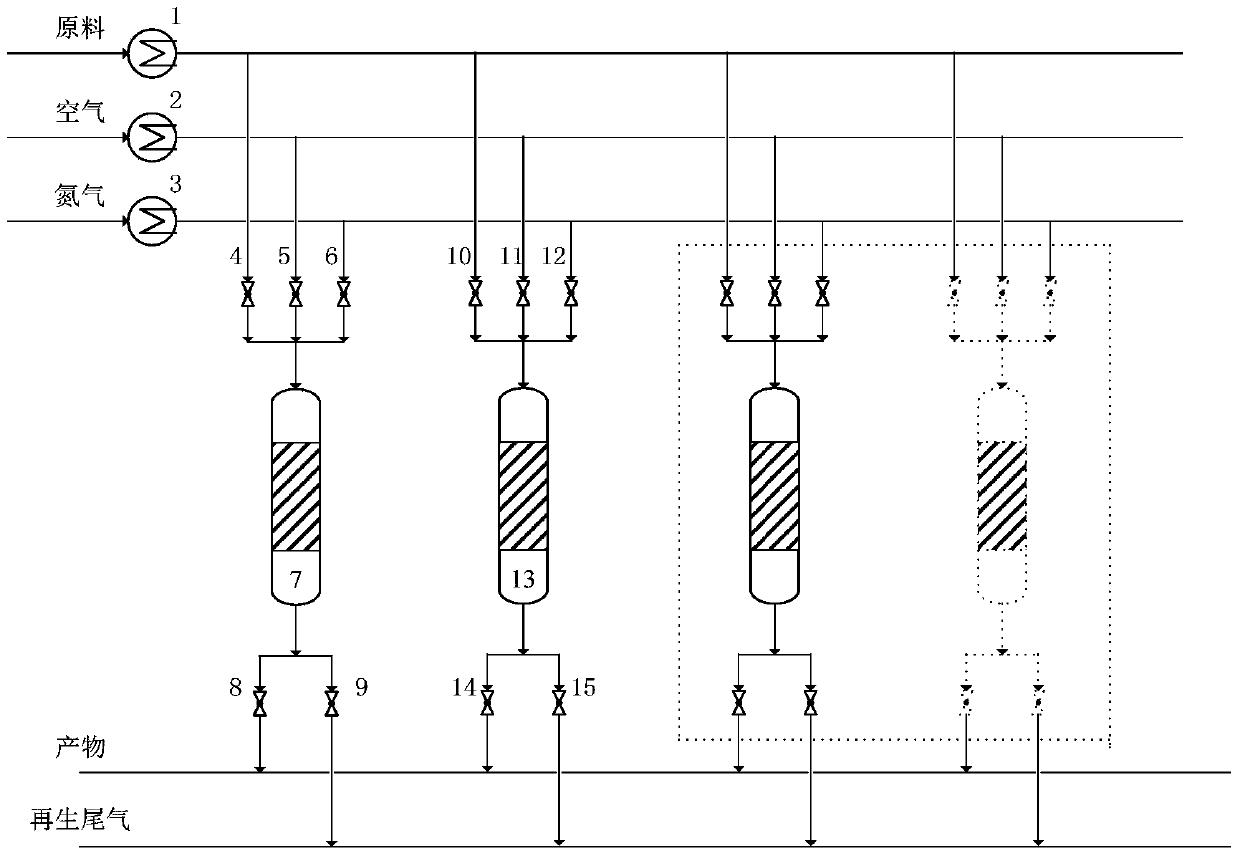
Image
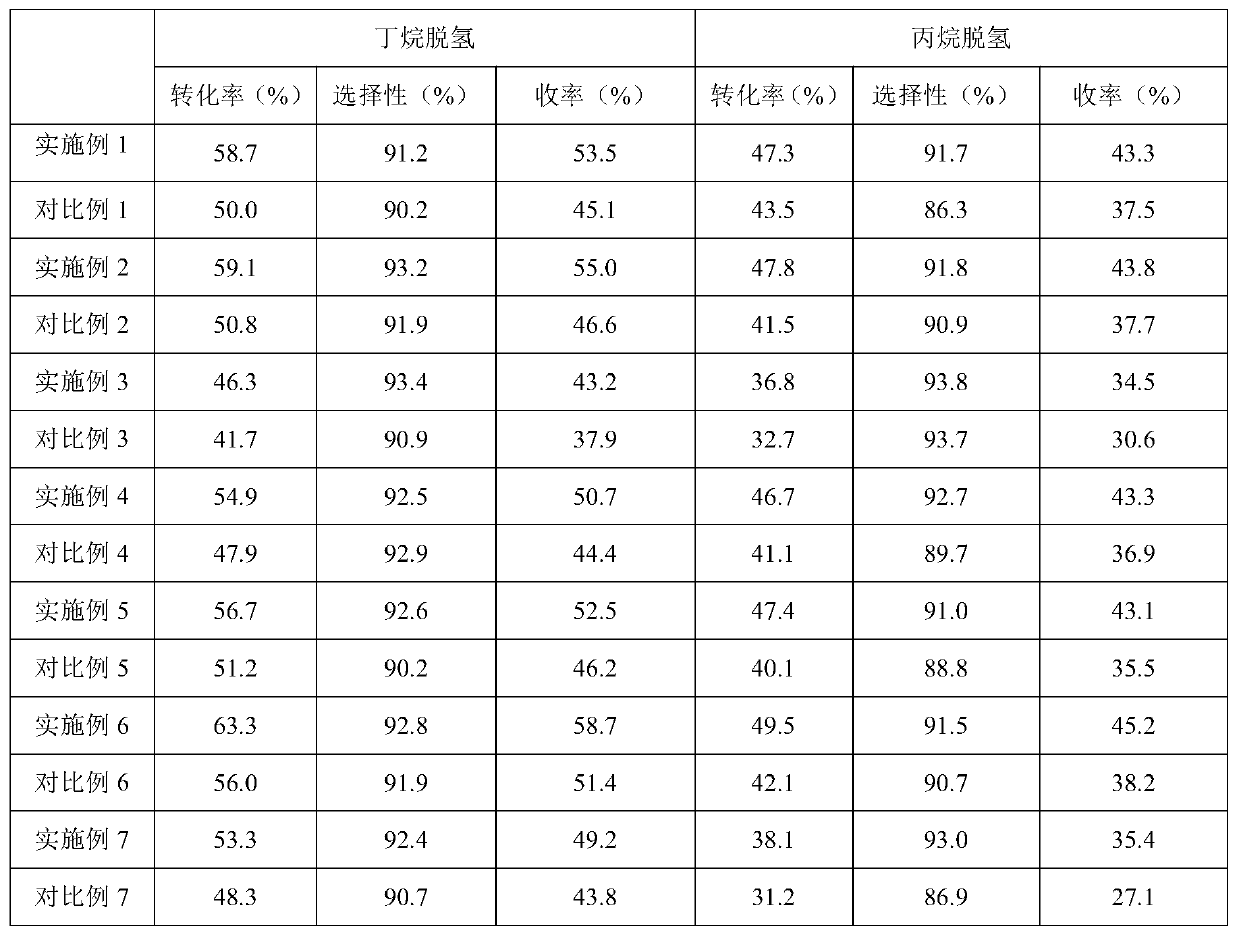
Examples
Embodiment 1
[0040] 223g (NH 4 ) 2 Cr 2 o 7 Dissolve in 800ml ethylene glycol, impregnate equal volume to 1000g porous γ-Al 2 o 3 in powder. The powder was placed in an oven at 120°C for 4 hours, washed with deionized water, filtered, and dried at 80°C for 2 hours. 37g KNO 3 Dissolved in 800ml of deionized water, impregnated into the above treated powder with equal volume, dried at 80°C for 4 hours, calcined at 680°C for 5 hours, and the catalyst obtained by forming and sieving was designated as Catalyst A.
[0041] Butane dehydrogenation:
[0042] Reaction process conditions, reaction temperature 590°C, pressure 0.11MPa, butane mass space velocity 1.0h -1 , the filling amount of the inert carrier is 100wt.% of the filling amount of catalyst A, the reaction time is 0.7h; the regeneration gas is air, the regeneration temperature is 660°C, the regeneration pressure is 0.10MPa, and the volume space velocity is 1000h -1 , regeneration time is 0.6h, nitrogen stripping space velocity is...
Embodiment 2
[0052] 177g CrO 3 , 248g (NH 4 ) 2 C 2 o 4 Dissolve in 800ml deionized water, impregnate equal volume into 1000g porous γ-Al 2 o 3 in powder. The powder was placed in an oven at 60°C for 6 hours, washed with deionized water, filtered, and dried at 100°C for 2 hours. 78gCu(NO 3 ) 2 ·3H 2 O, 112g Fe(NO 3 ) 3 9H 2 O was dissolved in 700ml of deionized water, impregnated into the above-mentioned treated powder with an equal volume, dried at 120°C for 3 hours, calcined at 700°C for 6 hours, and the catalyst obtained by forming and sieving was designated as Catalyst C.
[0053] Butane dehydrogenation:
[0054] Reaction process conditions, reaction temperature 585°C, pressure 0.11MPa, mass space velocity of butane 2.5h -1 , the loading amount of the inert carrier is 200wt.% of the loading amount of catalyst C, the reaction time is 0.5h; the regeneration gas is air, the regeneration temperature is 670°C, the regeneration pressure is 0.10MPa, and the volume space velocity...
Embodiment 3
[0064] 130g K 2 Cr 2 o 7 Dissolve in 800ml glycerol, impregnate equal volume into 1000g porous γ-Al 2 o 3 in powder. The powder was placed in an oven at 140°C for 1 hour, washed with deionized water, filtered, and dried at 80°C for 4 hours. 33g NaNO 3 , 82g Ni(NO 3 ) 2 ·6H 2 O was dissolved in 800ml of deionized water, impregnated into the above-mentioned treated powder with an equal volume, dried at 60°C for 6 hours, calcined at 720°C for 4 hours, and the catalyst obtained by forming and sieving was designated as Catalyst E.
[0065] Butane dehydrogenation:
[0066] Reaction process conditions, reaction temperature 610°C, pressure 0.2MPa, butane mass space velocity 0.5h -1 , the filling amount of the inert carrier is 50wt.% of the filling amount of the catalyst D, and the reaction time is 0.2h; the regeneration gas is air, the regeneration temperature is 650°C, the regeneration pressure is 0.10MPa, and the volume space velocity is 3000h -1 , regeneration time is 0....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com