Low-carbon alkane circulating fluidized bed dehydrogenation process
A circulating fluidized bed, low-carbon alkane technology, applied in the direction of hydrocarbons, hydrocarbons, carbon compound catalysts, etc., can solve the problem of high cost of tubular reactors, improve the anti-carbon performance, effectively provide, Produce continuous effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
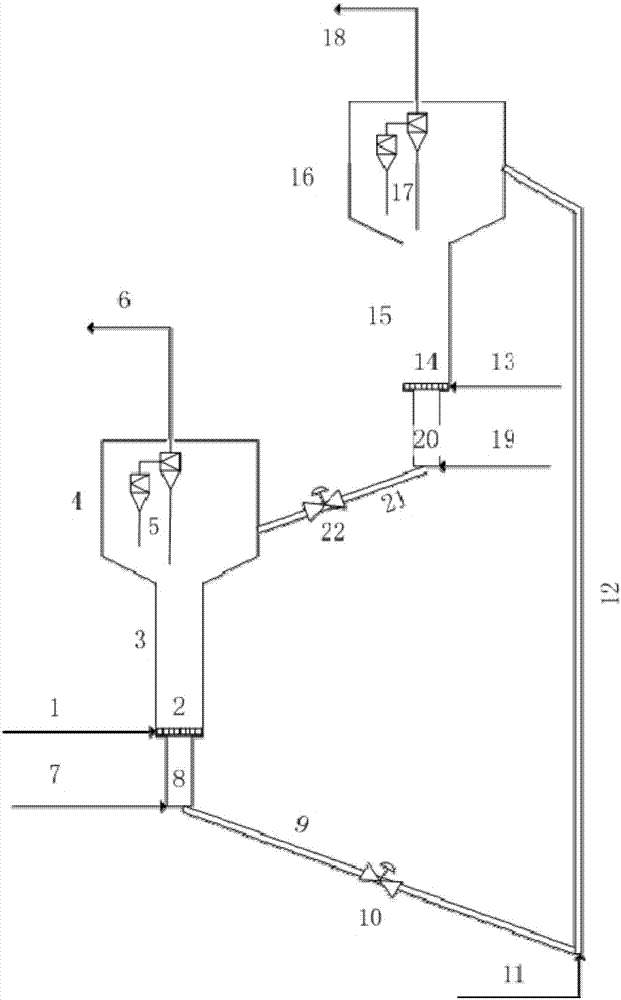
Image
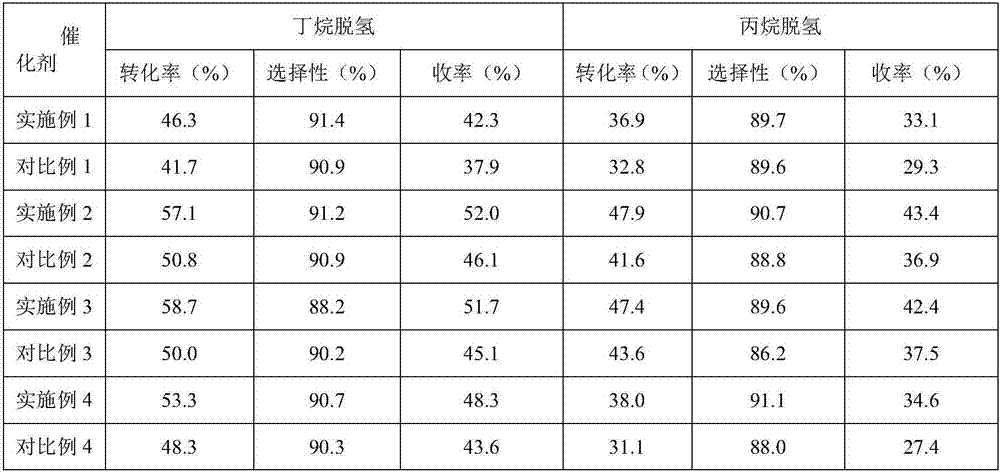
Examples
Embodiment 1
[0047] In a reactor heated by a water bath, add 50kg water, 4kg pseudo-boehmite and 1kg aluminum sol and stir evenly, acidify with 3kg nitric acid (analytical purity), heat up to 80℃, age for 4h; add 5kg macropore after cooling to room temperature Alumina is mixed uniformly and spray-dried to form, and calcined at 700°C for 6 hours to obtain a microsphere carrier.
[0048] 1.04kg K 2 Cr 2 O 7 Dissolved in 6.4L glycerol, immersed in an equal volume in 8kg microsphere carrier. Place it in an oven at 140°C for 1 hour, wash with deionized water, filter, and dry at 80°C for 4 hours. 0.26kg NaNO 3 , 0.66kg Ni(NO 3 ) 2 ·6H 2 O was dissolved in 6.4L deionized water, immersed in an equal volume in the above-treated microsphere carrier, dried at 60°C for 6 hours, and calcined at 720°C for 4 hours. The obtained catalyst was designated as catalyst A.
[0049] Butane dehydrogenation:
[0050] The reaction process conditions are as follows: reaction temperature is 610℃, pressure is 0.20MPa, cata...
Embodiment 2
[0061] In a water bath heated reactor, add 50kg water, 3kg pseudo-boehmite and 1kg aluminum sol, stir evenly, acidify with 2kg hydrochloric acid (analytical purity), heat to 70℃, age for 2h; add 8kg macropore after cooling to room temperature Alumina is mixed uniformly and spray-dried to form, and calcined at 550°C for 4 hours to obtain a microsphere carrier.
[0062] 1.77kg CrO 3 , 2.48kg(NH 4 ) 2 C 2 O 4 Dissolved in 8L of deionized water, immersed in 10kg of microsphere carrier in equal volume. Place it in an oven at 60°C for 6h, wash with deionized water, filter, and dry at 100°C for 2h. 0.78kg Cu(NO 3 ) 2 ·3H 2 O, 1.12kg Fe(NO 3 ) 3 · 9H2O was dissolved in 7L of deionized water, immersed in an equal volume in the above-treated microsphere carrier, dried at 120°C for 3 hours, and calcined at 700°C for 6 hours. The obtained catalyst was designated as catalyst C.
[0063] Butane dehydrogenation:
[0064] The reaction process conditions are as follows: reaction temperature is 595℃...
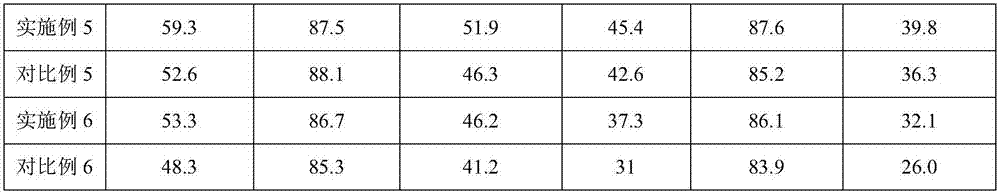
Embodiment 3
[0075] In a reactor heated by a water bath, add 50kg water, 4kg pseudo-boehmite and 3kg aluminum sol, stir evenly, acidify with 1kg hydrochloric acid (analytical purity), heat to 50℃, aging for 0.5h; add 8kg after cooling to room temperature The porous alumina is mixed uniformly and spray-dried to form, and calcined at 450°C for 2 hours to obtain the microsphere carrier.
[0076] 2.23kg(NH 4 ) 2 Cr 2 O 7 Dissolve in 8L ethylene glycol and immerse it in 10kg microsphere carrier in equal volume. Place it in an oven at 120°C for 4h, wash with deionized water, filter, and dry at 80°C for 2h. 0.37kg KNO 3 Dissolved in 8L of deionized water, immersed in an equal volume in the above-treated microsphere support, dried at 80°C for 4 hours, and calcined at 680°C for 5 hours. The obtained catalyst was designated as catalyst E.
[0077] Butane dehydrogenation:
[0078] Reaction process conditions: reaction temperature is 585℃, pressure is 0.10MPa, catalyst-oil mass ratio is 3, ratio of catalys...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com