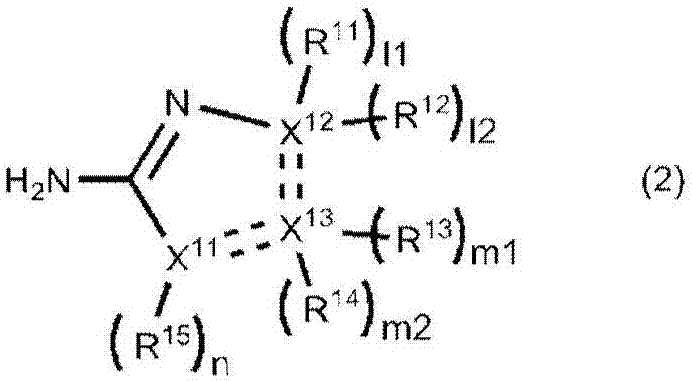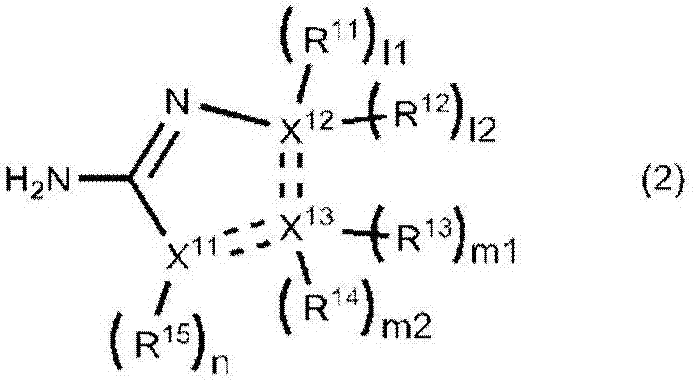Immunoassay method and assay reagent used in said method
A technology for antibody detection and samples, applied in biological testing, measuring devices, material inspection products, etc., can solve problems such as the difference between the measured value and the real value, interference hindering the detection of antigens and antibodies, and damage to the accuracy of the measured value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0179] [Example 1] Confirmation of non-specific reaction inhibitory effect of BPF on samples with L-FABP concentration near normal value, part 1
[0180] The non-specific reaction inhibitory effect in the L-FABP concentration range around the normal value when no protein (Comparative Example 1), BSA (Comparative Example 1) or 0.1% BPF (Example 1) was added to the first reagent was compared.
[0181] 1. Operation
[0182] (1) Embodiment 1
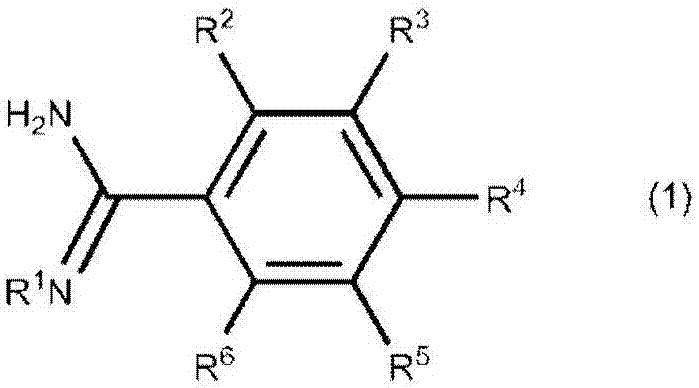
[0183] Using frozen-thawed urine as a sample, L-FABP in the sample was determined by using a first control reagent containing 390 mmol / L of the compound of formula (1) (benzamidine hydrochloride) and 0.1% BPF and a second reagent.
Embodiment 2 to 5
[0193] [Examples 2 to 5] Confirmation of non-specific reaction inhibitory effect of BPF on samples having L-FABP concentrations near normal values, part 2
[0194] Compare 390mmol / L compound (benzamidine hydrochloride) of formula (1) and 0.01% to 1% BPF (embodiment 2 to 5) when being added to the nonspecific in the L-FABP concentration range near the normal value when the first reagent Heterosexual response suppression effect.
[0195] 1. Operation
[0196] For the samples, a portion of urine (n=10; measured value in the reference method 0.6 ng / mL to 4.9 ng / mL) frozen at -30°C after collection was thawed only once and used for measurement. The amount of BPF indicated in Table 2 was added to the first reagent. Example 2 was performed in the same manner as Example 1 except these points.
[0197] 2. Results
[0198] The net absorbances of the samples measured in the experiments of Examples 2 to 5 were converted into L-FABP concentrations by using a calibration curve establish...
Embodiment 6 to 9
[0202] [Examples 6 to 9] Confirmation of non-specific reaction inhibitory effect of BPF on samples having L-FABP concentrations near normal values, part 3
[0203] Compare 390mmol / L compound (2-amino-2-thiazoline hydrochloride) of formula (2) and 0.01% to 1% BPF (embodiments 6 to 9) when adding to the first reagent near the normal value L-FABP Inhibitory effect of non-specific reactions in the concentration range.
[0204] 1. Operation
[0205] For the samples, a portion of urine (n=10; measured value in the reference method 0.6 ng / mL to 4.9 ng / mL) frozen at -30°C after collection was thawed only once and used for measurement. The compound of formula (2) (2-amino-2-thiazoline hydrochloride) and the amount of BPF shown in Table 3 were added to the first reagent. Example 3 was performed in the same manner as Example 1 except these points.
[0206] 2. Results
[0207] The net absorbances of the samples measured in the experiments of Examples 6 to 9 were converted into L-FABP ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com