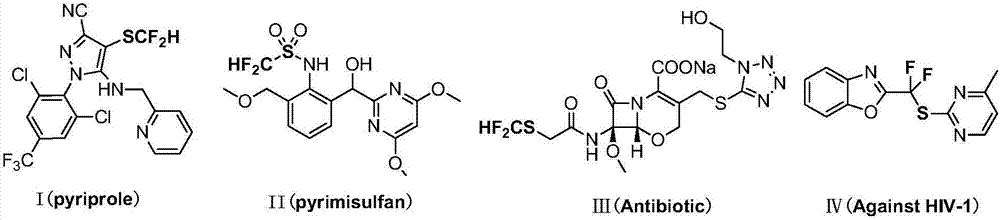
Preparation method for N-difluoromethylthiophthalimide compound
A technology of difluoromethylthio-o- and difluoromethylthio, which is applied in the field of preparing N-difluoromethylthio-phthalimide compounds, and can solve the problem of harsh reaction conditions, low conversion rate, and inapplicability to industrial production And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0068] Example 1 N-difluoromethylthio-phthalimide 3
[0069]
[0070] 1) Preparation of difluoromethyl-substituted benzylthiol 2
[0071] method one,
[0072] Add n-hexane (1500mL) into the three-necked flask, place it in a -78°C cold bath and stir, slowly pass through chlorodifluoromethane (F-22, 750mmol), add NaOH (50g, 1250mmol) at -78°C, three (3,6-dioxaheptyl)amine (TDA-1, 8.1g, 25mmol) and benzyl mercaptan (62.1g, 500mmol) were reacted slowly at 60°C for 2-4h under dry ice-acetone condensation. The reaction solution was filtered, subjected to desolvation under reduced pressure, and separated by flash silica gel column chromatography to obtain 53 g of light pink oily liquid with a yield of 61%. The hydrogen spectrum showed a purity greater than 98%.
[0073] Method Two,
[0074] Add KOH (168g, 3000mmol), acetonitrile / water (1400mL, 1:1) and benzyl mercaptan (18.6g, 150mmol) into a three-necked flask, stir in a cold bath at -78°C, and add bromodifluorophosphoric acid...
Embodiment 2
[0083] Example 2 N-difluoromethylthio-4-nitro-phthalimide 3
[0084]
[0085] Add 98mLCl to the three-necked flask 2 / CHCl 3 solution (1.232mol / L, 120mmol), the flask was placed in a -10°C cold bath and stirred, and difluoromethyl-substituted benzylthiol 2 (20.9g, 120mmol) was added, and the reaction was carried out for 1h (using 19 F NMR for monitoring). Under a cooling bath at -10°C, 4-nitrophthalimide potassium salt (27.6 g, 120 mmol) was added rapidly, and then raised to room temperature for 10 h. The reaction solution was filtered, subjected to desolvation under reduced pressure, and separated by flash silica gel column chromatography to obtain 19.7 g of a white solid with a yield of 60%. The hydrogen spectrum showed that the purity was greater than 98%.
[0086]
[0087] N-difluoromethylthio-4-nitrophthalimide (N-(difluoromethylthio)-4-nitrophthalimide): 1 H NMR (400MHz, CDCl 3 ,293K,TMS)δ8.82(d,J=5.2Hz,1H),8.74(dd,J=8.2,1.8Hz,1H),8.24(d,J=8.2Hz,1H),6.80(t,J ...
Embodiment 3
[0088] Example 3 N-trifluoromethylthio-phthalimide
[0089]
[0090] Method 1: At 0°C, add CF into 1,2-dichloroethane of o-benzimide potassium salt (3.7g, 20mmol) 3 SCl gas, then react the reaction solution at room temperature (25°C) for 7h (with a reflux condenser at -20°C), filter after the reaction, and recrystallize the filtrate to obtain 0.5g of a white solid with a yield of 10 %, the hydrogen spectrum shows that the purity is greater than 98%.
[0091] Method 2: At 0°C, add CF into 1,2-dichloroethane of o-benzimide potassium salt (3.7g, 20mmol) 3 SCl gas, then the reaction solution was reacted at 90-100°C for 7h (with a -20°C reflux condenser), after the reaction was completed, it was cooled to room temperature, filtered, and the filtrate was precipitated and recrystallized to obtain 3.2g of a white solid. The yield is 65%, and the hydrogen spectrum shows that the purity is greater than 98%.
[0092] N-(trifluoromethylthio)phthalimide: 1 H NMR (400MHz, CDCl 3 )δ8...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com