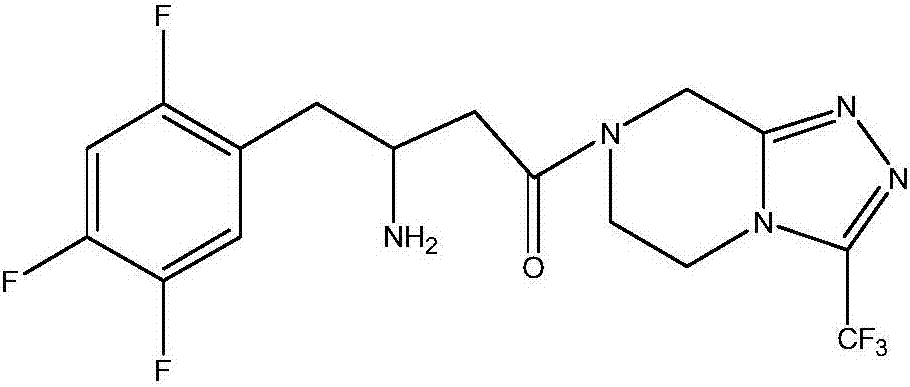
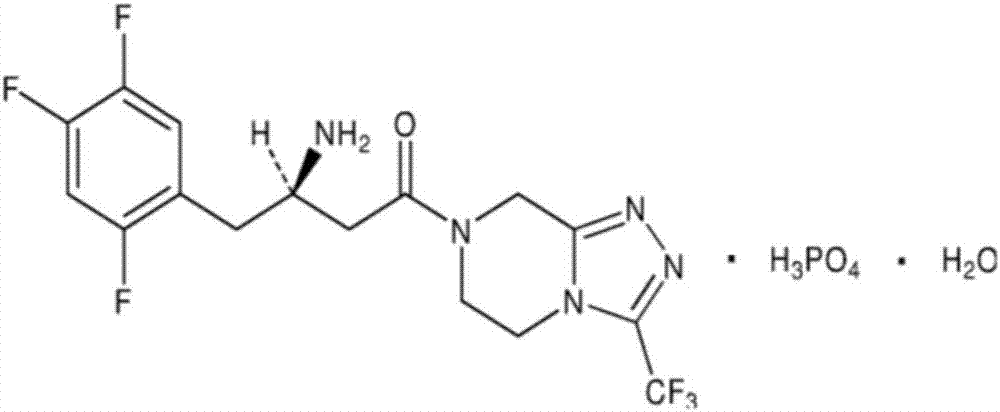
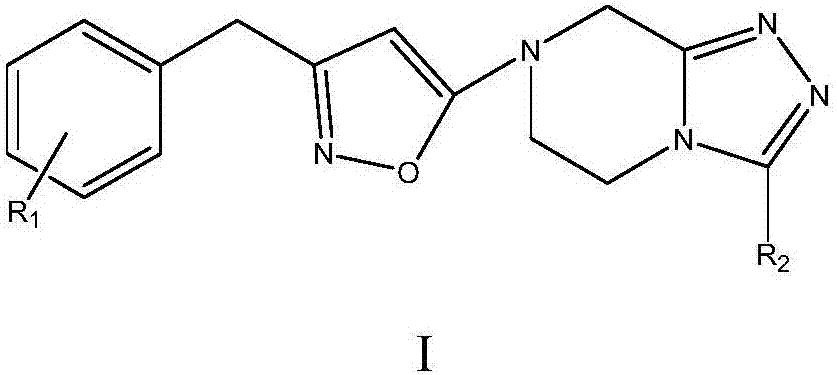
Sitagliptin derivative or pharmaceutically acceptable salt, as well as preparation method and application thereof
A derivative and pharmaceutical technology, applied in the field of synthesis of pharmaceutical intermediates, can solve problems such as low-energy molecular conformation, reduced drug selectivity, unfavorable safe drug use, etc., to reduce the formation of low-energy isomerism, improve selectivity, and improve drug safety sexual effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Preparation of enamine key intermediate formula II compound:
[0054]
[0055] Add dichloromethane (20mL) in the reaction flask equipped with stirring, condenser, thermometer, formula VI compound (0.01mol), namely wherein R 3 For hydrogen, tert-butyl carbamate (1.76g, 0.015mol), p-toluenesulfonic acid (0.1g) and MS type molecular sieves (3.0g), stirred overnight at room temperature, after the reaction was completed, the reaction solution was filtered, The filtrate was concentrated to dryness under reduced pressure, then purified by silica gel column chromatography, and the purified eluate was collected and then concentrated to dryness under reduced pressure to obtain an off-white solid compound enamine key intermediate formula II compound with a yield of more than 60%. .
[0056] For the synthesis of the target compound in the specific synthesis process, the substituent R in the molecular structure of the corresponding compound 1 Selective replacement is enough to ...
Embodiment 2
[0060] Preparation of intermediate formula IV compound
[0061]
[0062] Select the corresponding enamine key intermediate formula II compound (3.4mmol) obtained in the above-mentioned embodiment 1 and add in the reaction flask, then, add the dichloromethane (50mL) solution of the corresponding formula III compound (3.4mmol) again, control the temperature at Add HOBT (545mg, 4.2mmol) at about 0°C, stir for 10 minutes, then add EDC (1.9g, 10.0mmol), then remove the ice bath, and slowly raise the temperature to room temperature under stirring to carry out the coupling reaction 14 Hours, after the reaction was over, the compound was concentrated, subjected to silica gel column chromatography, and the pure components were combined and then concentrated to dryness under reduced pressure to obtain an off-white solid intermediate compound of formula IV.
[0063] For the difference in the target compound intermediate formula IV compound in the specific synthetic process, the substi...
Embodiment 3
[0069] Preparation of intermediate formula IV-1 compound
[0070]
[0071] Select the corresponding enamine key intermediate compound (3.4mmol) obtained in the above-mentioned embodiment 1 and add it to the reaction flask, and then add the corresponding compound 3-(trifluoromethyl)-5,6,7,8 -Tetrahydro-[1,2,4]triazolo[4,3-a]pyrazine (3.4mmol) in dichloromethane (50mL) solution, add HOBT (545mg, 4.2mmol), stirred for 10 minutes, then added EDC (1.9g, 10.0mmol), then removed the ice bath, slowly warmed up to room temperature under stirring conditions, and stirred for 14 hours for coupling reaction. After the reaction was over, concentrated the reaction solution to remove Solvent, the corresponding residue was obtained, after silica gel column chromatography, the pure components were combined and then concentrated to dryness under reduced pressure to obtain 1.3 g of an off-white solid intermediate compound of formula IV-1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Yield | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com