Combination of capric acid and prostaglandin e2 as auxiliary diagnostic markers for macrosomia and its application
An auxiliary diagnosis and prostaglandin technology, which is applied in the field of analytical chemistry and clinical medicine, can solve the problems of complex experimental steps and low sensitivity, and achieve the effects of improving sensitivity and specificity, easy detection, delaying and preventing disease progression
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0093] Embodiment 1 Research Object Selection and Grouping Basis
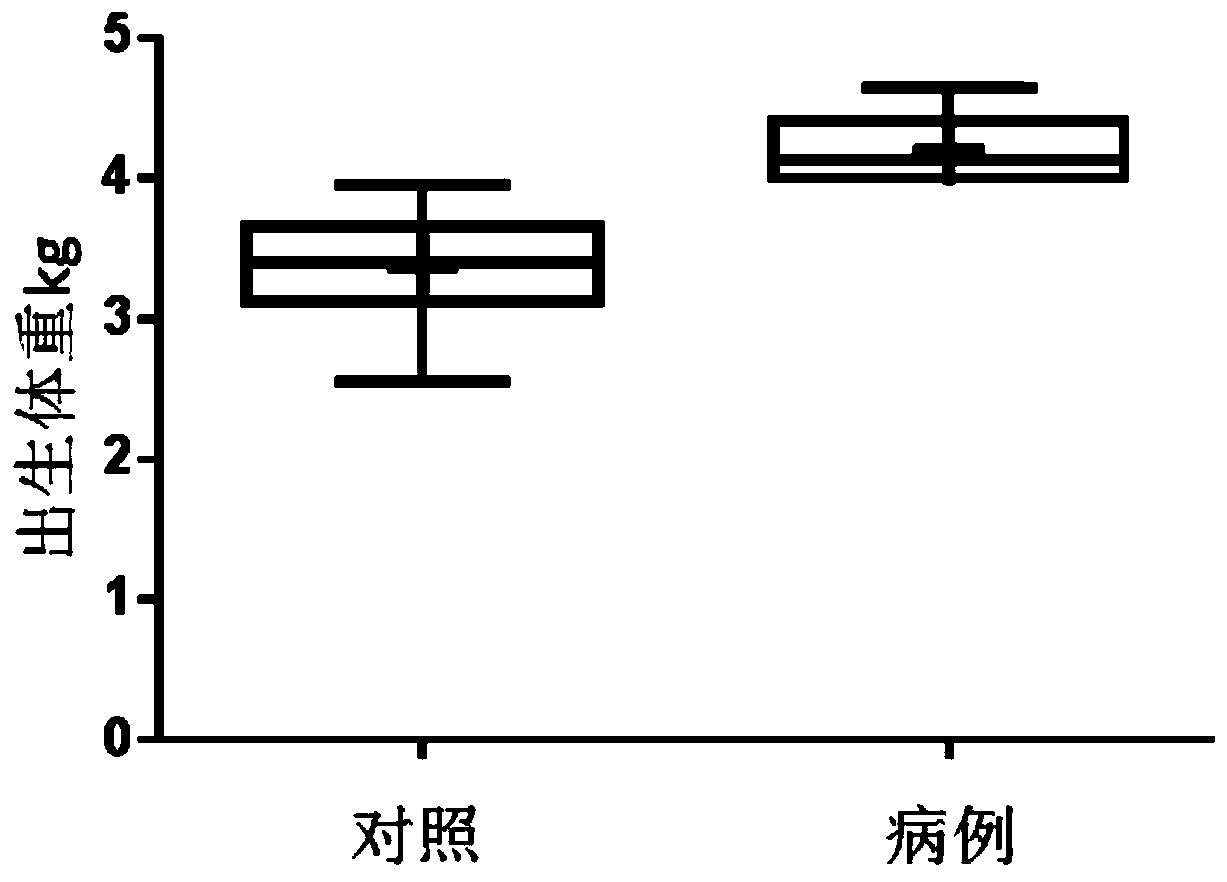
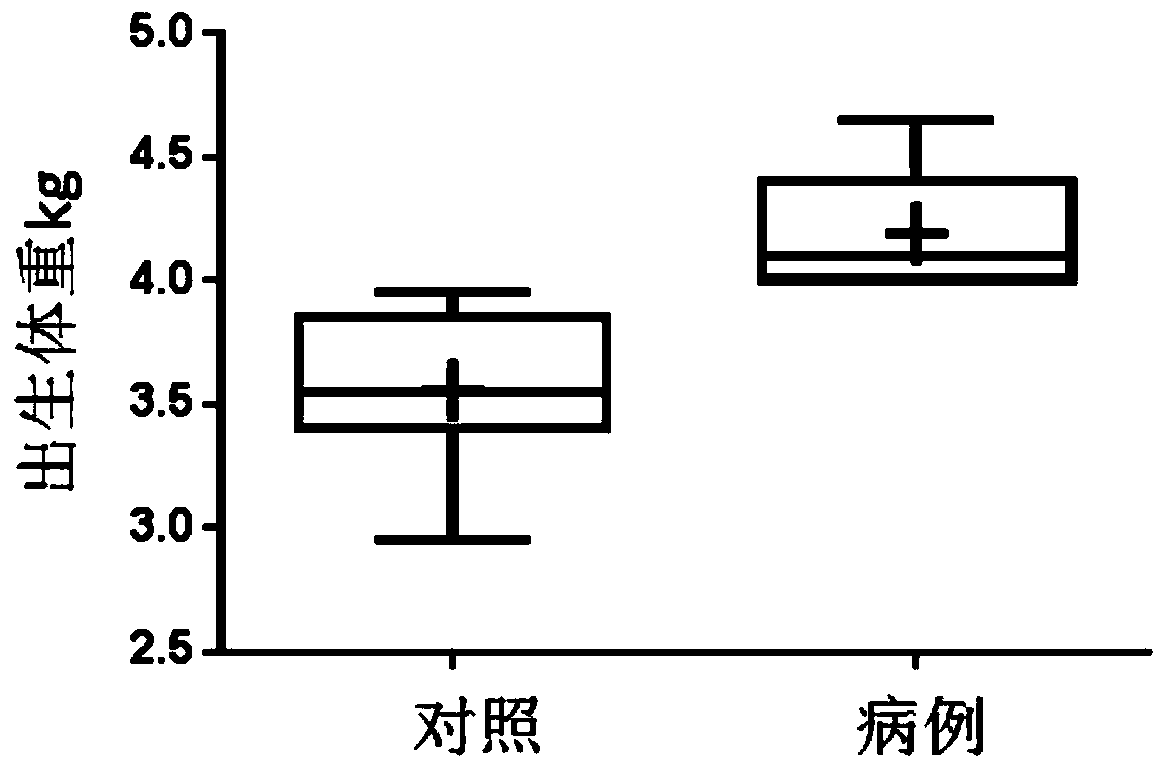
[0094] The present inventor collected blood samples from mothers of macrosomia patients and normal children who met the requirements from the Nanjing Maternal and Child Health Hospital Affiliated to Nanjing Medical University (see figure 1 , figure 2 ). Weigh the newborn after birth, and if the weight is equal to or greater than 4kg within 1 hour after birth, it is diagnosed as "macrosomia". In the first stage, 97 samples of pregnant women who met the requirements were randomly included, 15 of which were macrosomia; in the second stage, 30 samples of pregnant women were included, 15 of which were compared with 15 cases of macrosomia, as the blood metabolism of small molecular organisms during pregnancy of macrosomia. Screening subjects for markers. Specific sample classification criteria are as follows:
[0095] first stage screening
[0096] A total of 97 pregnant women were randomly included.
[0097] ...
Embodiment 2
[0110] Example 2: UPLC-MS Metabolomics Biomarker Screening for Macrosomia
[0111] 1. Sample pretreatment
[0112] 1.1. Centrifuge fresh pregnant blood at 3000rpm for 5min, take 100μL supernatant and put it into a clean 1.5ml EP tube.
[0113] 1.2. Add 50 μL of water to 50 μL of supernatant and add 300 μL of methanol (reagent A) to precipitate protein.
[0114] 1.3. Aspirate the supernatant, blow dry with nitrogen and then vacuum dry.
[0115] 1.4. Reconstitute with 20 μL of the dried product (reagent D).
[0116] 2. Instrument testing
[0117] 2.1. Analytical instruments: UPLC Ultimate 3000 system (Dionex) high-performance liquid chromatography; Q-Exactive high-resolution mass spectrometer.
[0118] 2.2. Liquid phase conditions:
[0119] 2.2.1. The liquid chromatographic column is a Hypersil GOLD C18 chromatographic column (100mm×2.1mm, particle size 1.9μm, Thermo Scientific, Germany), and the column temperature is 40°C.
[0120] 2.2.2 Mobile phase A is water containing...
Embodiment 3
[0132] Example 3 Stability Analysis of Blood Metabolism Small Molecules
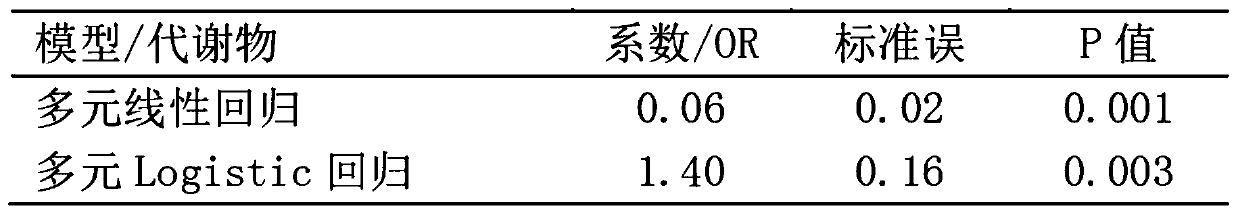
[0133] The method of Example 2 was used to evaluate the stability of the combination level of capric acid and prostaglandin E2 in the mother's blood during pregnancy (the interval was 2 weeks). The results showed that combined capric acid and prostaglandin E2 levels in blood were stable ( Figure 4 ), with properties as diagnostic / monitoring markers.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com