Cefuroxime sodium crystal compound and preparation method thereof
A crystalline compound, the technology of cefuroxime sodium, applied in the field of medicine, can solve problems such as poor stability, and achieve the effects of good stability, high yield and good fluidity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
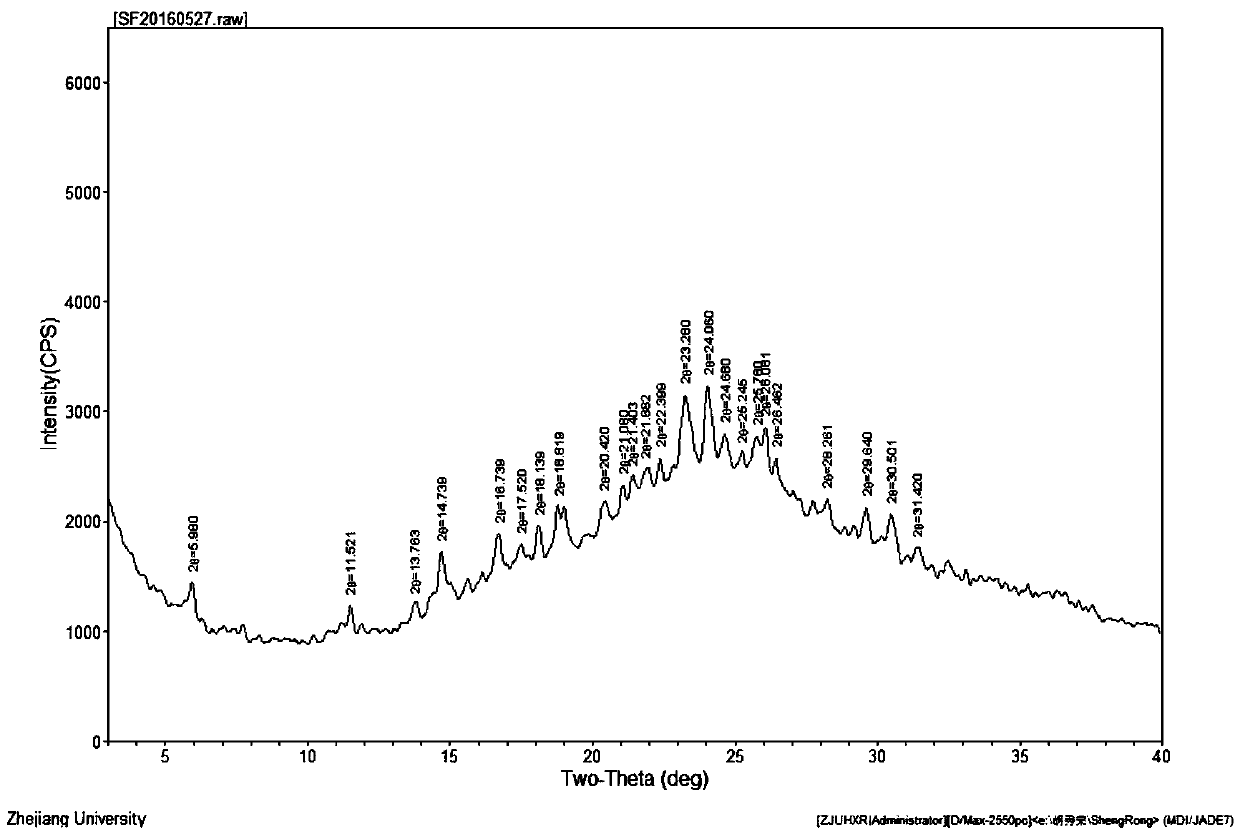
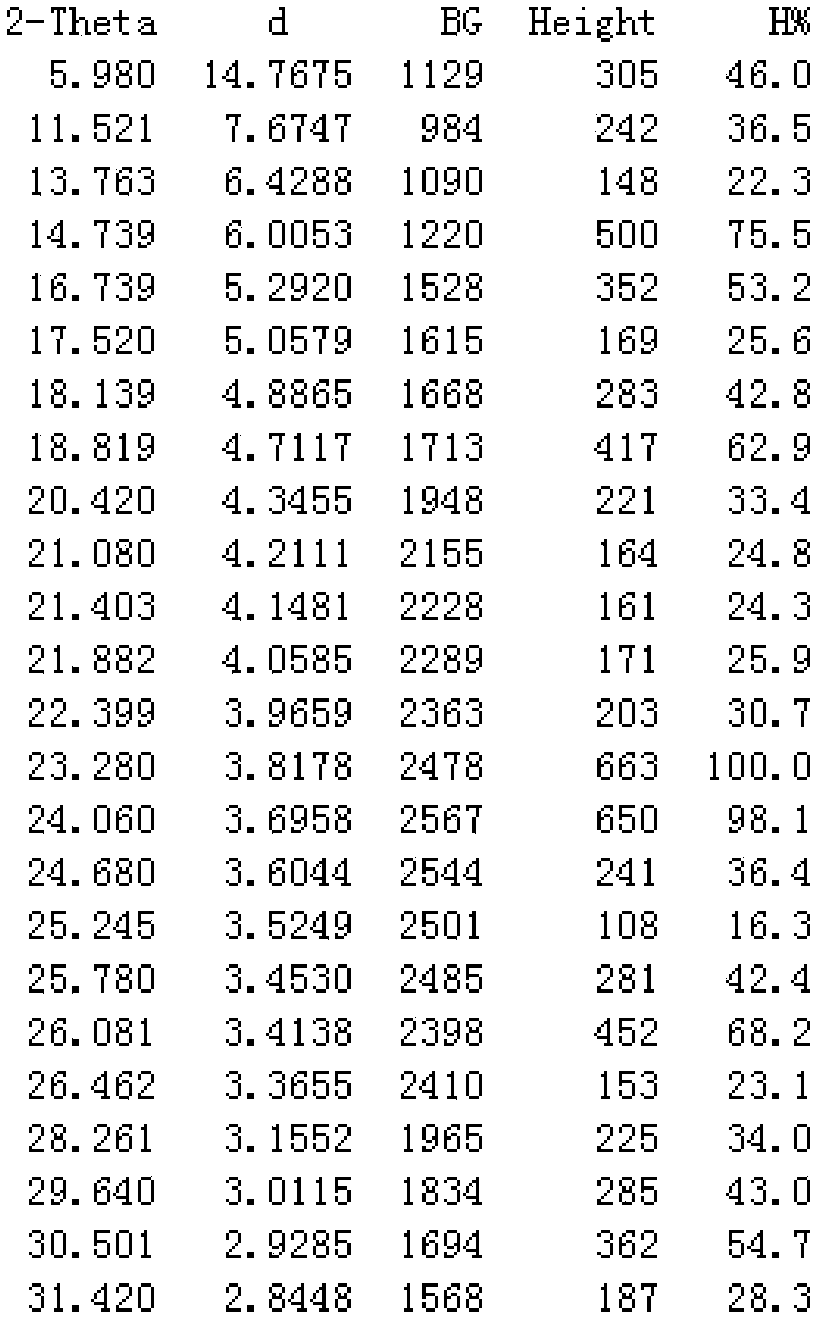
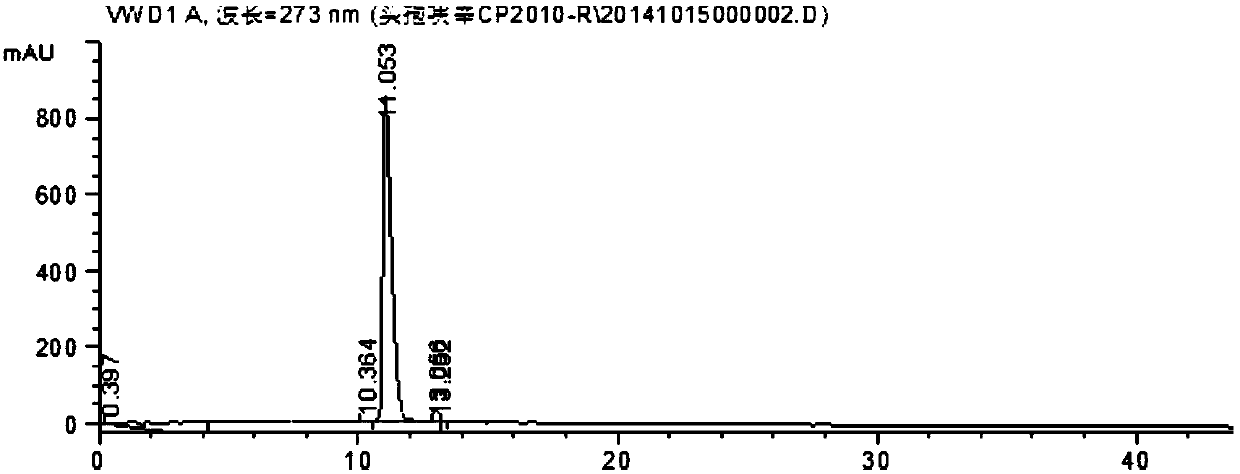
Image
Examples
Embodiment 1
[0024] In a 5000mL flask, dissolve 446g (1mol) of crude cefuroxime sodium in 800mL of water, add 2000ml of acetone at 25°C, place the flask in an ultrasonic generator, control the temperature at 15°C, turn on the ultrasonic seeding for 30min, and The frequency is 50KHz, then stir for 30min, then slowly add 1000mL acetone to the flask, control the temperature at 10°C for crystallization for 2h, filter, wash the filter cake twice with 500mL of acetone, vacuum dry the material at 20°C for 30min, then rise to 45°C Cefuroxime sodium was dried under vacuum for 3 hours to obtain 431 g of the crystalline compound of cefuroxime sodium, with a yield of 96.7%.
Embodiment 2
[0026] In a 5000mL flask, dissolve 446g (1mol) of crude cefuroxime sodium in 800mL of methanol, add 2000ml of acetone at 25°C, place the flask in an ultrasonic generator, control the temperature at 15°C, and turn on the ultrasonic seeding for 30min. The ultrasonic frequency is 50KHz, then stir for 30min, then slowly add 1000mL acetone to the flask, control the temperature at 10°C for crystallization for 2h, filter, wash the filter cake twice with 500mL of acetone, vacuum dry the material at 20°C for 30min, and then rise to After vacuum drying at 45°C for 3 hours, 44.6 g of the crystalline compound of cefuroxime sodium was obtained, with a yield of 10%.
Embodiment 3
[0028] In a 5000mL flask, dissolve 446g (1mol) of crude cefuroxime sodium in 800mL of water, add 2000ml of ethanol at 25°C, place the flask in an ultrasonic generator, control the temperature at 15°C, turn on ultrasonic seeding for 30min, and The frequency is 50KHz, then stir for 30min, then slowly add 1000mL ethanol to the flask, control the temperature at 10°C for crystallization for 2h, filter, wash the filter cake twice with 500mL ethanol each, vacuum dry the material at 20°C for 30min, then rise to 45°C Cefuroxime sodium was dried under vacuum for 3 hours to obtain 138 g of the crystal form compound of cefuroxime sodium, with a yield of 31%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com