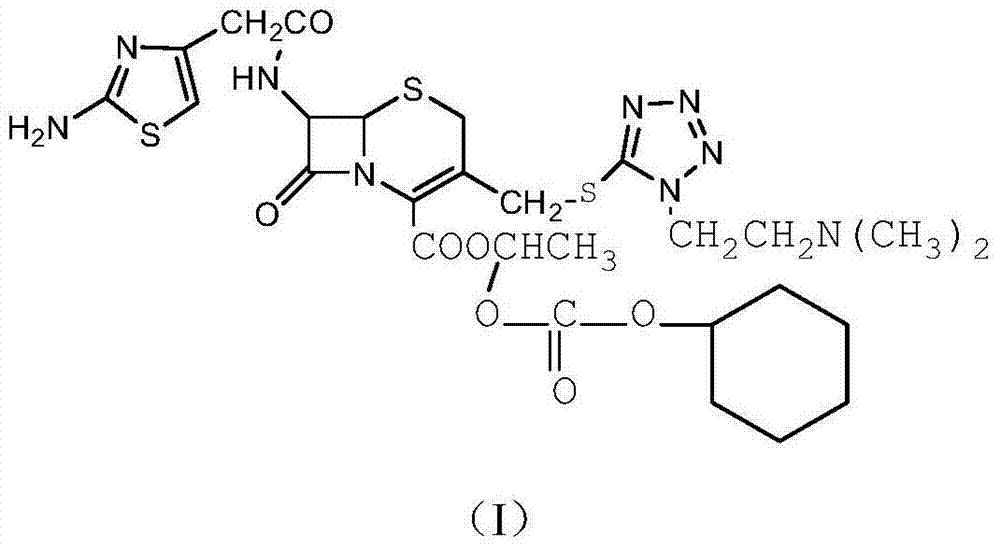
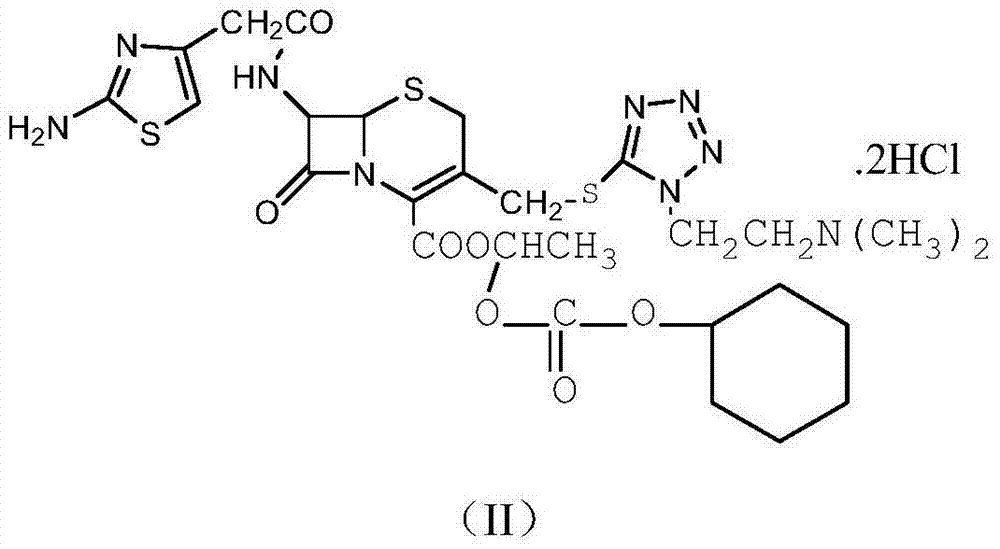
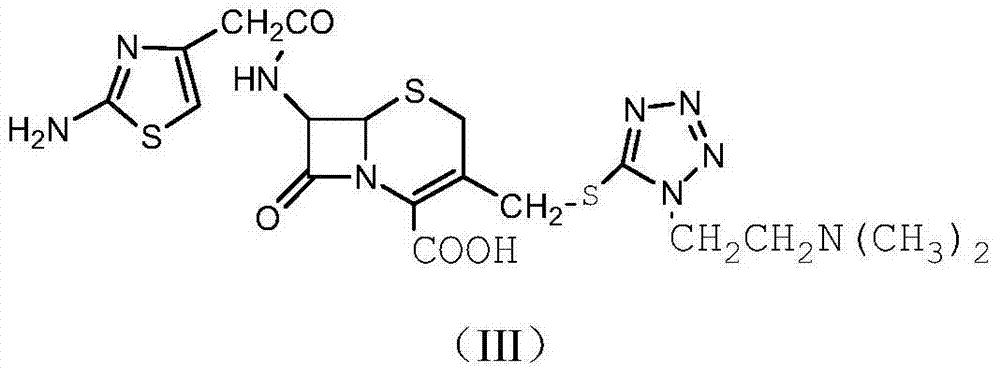
The refining method of cefotiam hydrochloride
A technology of cefotiam pivoxil and a purification method, applied in the field of medicine, can solve the problems of difficulty in filtration, influence product quality, small product particle size, etc., and achieve simple and easy post-processing, high product quality, and uniform crystal particle size distribution. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] (1) In a three-neck flask, dissolve 1.5 mol of sodium carbonate in 600 mL of distilled water, then add 1 mol of cefotiam hydrochloride, stir at room temperature, and react for 1 hour to obtain cefotiam sodium salt.
[0034] (2) Add 1 mol of the cefotiam sodium salt obtained above and 1.5 mol of 1-iodoethyl cyclohexyl carbonate to 400 mL of dimethylformamide solvent, the reaction temperature is 10 ° C, the reaction time is 12 min, and water is added under stirring cool down.
[0035] (3) 1000 ml of ethyl acetate was added to the obtained reaction solution for extraction.
[0036] (4) Transfer the organic phase extracted in step (3) to a 5000ml three-neck bottle, place it in an ultrasonic generator, control the temperature at 15°C, and add 5 parts by volume of a mixed solvent under stirring (the mixed solvent is a mass fraction of 3% HCl and 97% acetone), after dropping, turn on ultrasonic seeding for 40min, the ultrasonic frequency is 60KHz, and then stir for 25min, the...
Embodiment 2
[0038] (1) In a three-neck flask, dissolve 2 mol of sodium bicarbonate in 700 mL of distilled water, then add 1 mol of cefotiam hydrochloride, stir at room temperature, and react for 0.5 h to obtain cefotiam sodium salt.
[0039] (2) Add 1 mol of the cefotiam sodium salt obtained above and 2 mol of 1-iodoethyl cyclohexyl carbonate to 600 mL of dimethylformamide solvent, the reaction temperature is 15 °C, the reaction time is 10 min, and cooled with water under stirring .
[0040] (3) Add 1500ml of methanol to the obtained reaction solution for extraction.
[0041] (4) Transfer the organic phase extracted in step (3) to a 5000ml three-neck bottle, place it in an ultrasonic generator, control the temperature at 10°C, and add 4 parts by volume of a mixed solvent under stirring (the mixed solvent is a mass fraction of 0.5% HCl and 99.5% acetone), after dripping, turn on the ultrasonic generator for seeding for 50min, the ultrasonic frequency is 20KHz, and then stir for 20min, the...
Embodiment 3
[0043] (1) In a three-neck flask, dissolve 1.8 mol of sodium isooctanoate in 700 mL of distilled water, then add 1 mol of cefotiam hydrochloride, stir at room temperature, and react for 0.8 h to obtain cefotiam sodium salt.
[0044] (2) Add 1 mol of the cefotiam sodium salt obtained above and 1.2 mol of 1-iodoethyl cyclohexyl carbonate to 500 mL of dimethylformamide solvent, the reaction temperature is -9 ° C, the reaction time is 20 min, under stirring Cool with water.
[0045] (3) Add 1200ml of dichloromethane to the obtained reaction solution for extraction.
[0046](4) Transfer the organic phase extracted in step (3) to a 5000ml three-neck bottle, place it in an ultrasonic generator, control the temperature at 25°C, and add 3 parts by volume of a mixed solvent under stirring (the mass fraction of the mixed solvent is 4% HCl and 96% methanol), after dripping, turn on the ultrasonic generator to seed the crystal for 30min, the ultrasonic frequency is 80KHz, then stir for 35...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com