Medicine composition for treating gynecological inflammation, and application, kit and package part
A technology for gynecological inflammation and a kit, which is applied in the field of pharmaceutical compositions for gynecological inflammation, can solve the problems of long-term use of suppositories, inconvenience of long-term use, slow onset of effect, and long cycle, and achieve excellent therapeutic effects and less toxic and side effects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] The pharmaceutical composition preparation that embodiment 1 hemorrhoids and Shuanghuangxiaoyan make
[0029] Prescription: Honeysuckle 264g, Sophora flavescens 188g, Phellodendron 33g, Galla 99g, Cnidium 133g, Sweet potato vine 133g; Three needles 191g, Scutellaria baicalensis 64g
[0030]Preparation method: honeysuckle vine, flavescens, Phellodendron cortex, gallnut, cnidium, sweet potato vine six flavors, soak in water for 2 hours, decoct three times (8 times the amount of water each time), the first time for 3 hours, the second time for 2 hours, For the third time, 1 hour, the decoction was combined, filtered, and the filtrate was concentrated into a clear ointment with a relative density of 1.12-1.16 (80°C) to obtain Zhiji Qing ointment for later use; Scutellaria baicalensis was decocted three times with 8, 6, and 6 times the amount of water respectively. , 3 hours for the first time, 1 hour for the second time, 0.5 hours for the third time, combine the decoction, ...
Embodiment 2
[0033] The hemorrhoid preparation and the Shuanghuang anti-inflammatory preparation are made into kits / packages
[0034] preparation of formulations
[0035] Hemorrhoids lotion: 1000ml of lotion was prepared according to the quality standard WS-10496 (ZD-0496)-2002-2012Z, and the prescription: 264g of honeysuckle, 188g of sophora, 33g of Phellodendron chinensis, 99g of Galla gall, 133g of Cnidium chinensis, and 133g of sweet potato vine; Specifications: 20ml / bottle, 100ml / bottle. The crude drug content is 0.85g / ml.
[0036] Shuanghuang Xiaoyan Tablets: according to the quality standard WS 3 -B-0235-90-6 prepared 1000 tablets, of which prescription: three needles 1286g, baicalin 429g; specifications: 6 tablets / board, 12 tablets / board. Crude drug content: 1.7g / tablet.
[0037] Shuanghuang Xiaoyan Capsules: 1000 capsules prepared according to the quality standard YBZ09012006-2009Z, of which prescription: three needles 1286g, Scutellaria baicalensis 429; specifications: 6 cap...
experiment example 1
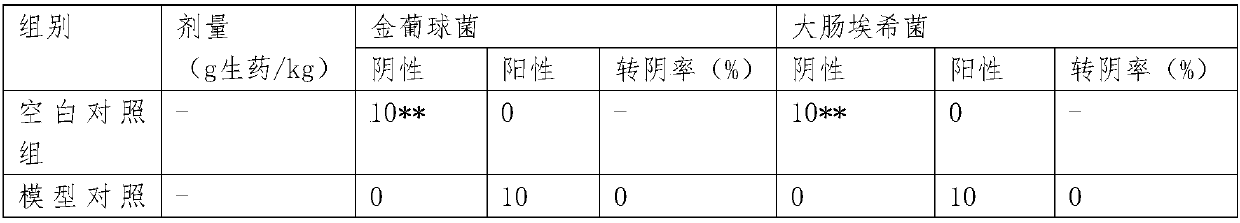
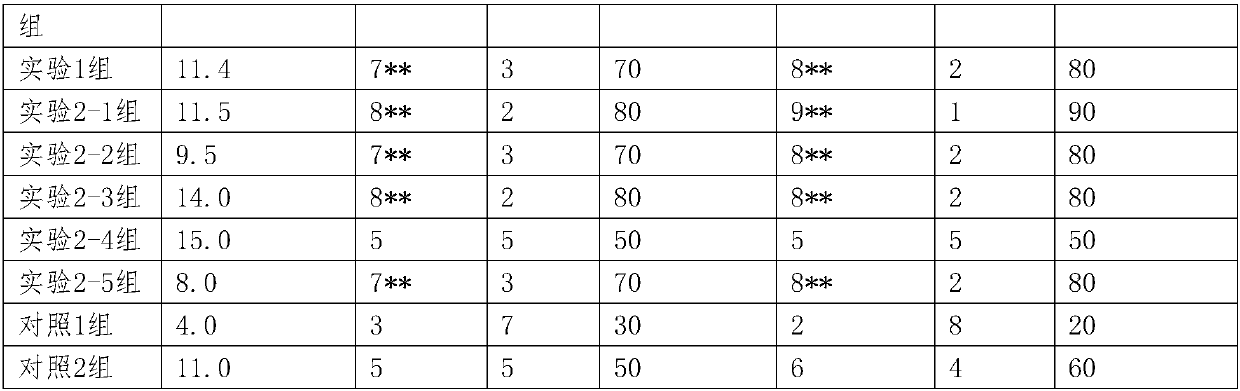
[0043] Experimental Example 1 Comparative observation of the anti-inflammatory, antibacterial and antipruritic effects of hemorrhoids combined with Shuanghuang Xiaoyan on experimental mice
[0044] 1.1 Materials
[0045] 1.1.1 Animals: Experimental animals: female Kunming mice, weighing 20-30g.
[0046] 1.1.2 Drug grouping and administration:
[0047] Experimental group 1: the pharmaceutical composition of hemorrhoids and Shuanghuang anti-inflammatory, prepared according to the method of Example 1, the crude drug content is 1.1g / ml; the clinical daily dosage for adults is 40ml, that is, 44g crude drug.
[0048] Experiment 2 groups: the test kit / package of hemorrhoid lotion+Shuanghuang Xiaoyan Tablets, prepared according to the method in Example 2, divided into: experiment 2-1 (hemorrhoids: 40ml is 34g crude drug, Shuanghuang Anti-inflammatory: 6 capsules or 10.2g crude drug), experiment 2-2 (hemorrhoid: 35ml or 29.75g crude drug, Shuanghuang anti-inflammatory: 4 capsules or ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com