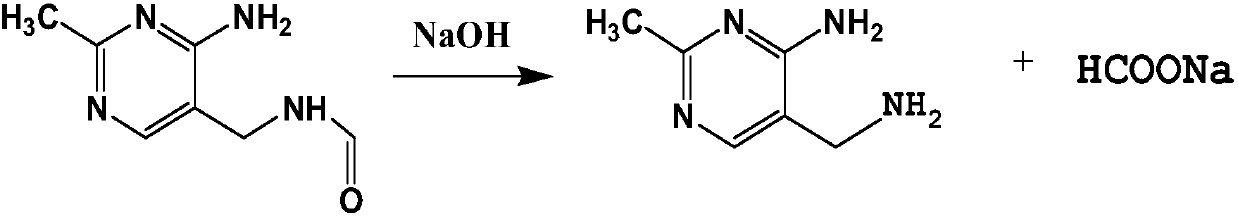
Hydrolysis process of 2-methyl-4-amino-5-(formamidomethyl)pyrimidine
A formamidomethyl and amino technology, which is applied in the field of preparation of vitamin B1 intermediates, can solve the problems of large waste water treatment volume, difficult separation, and difficult waste water treatment, and achieves reduction of difficulty, cost saving, and high product content. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
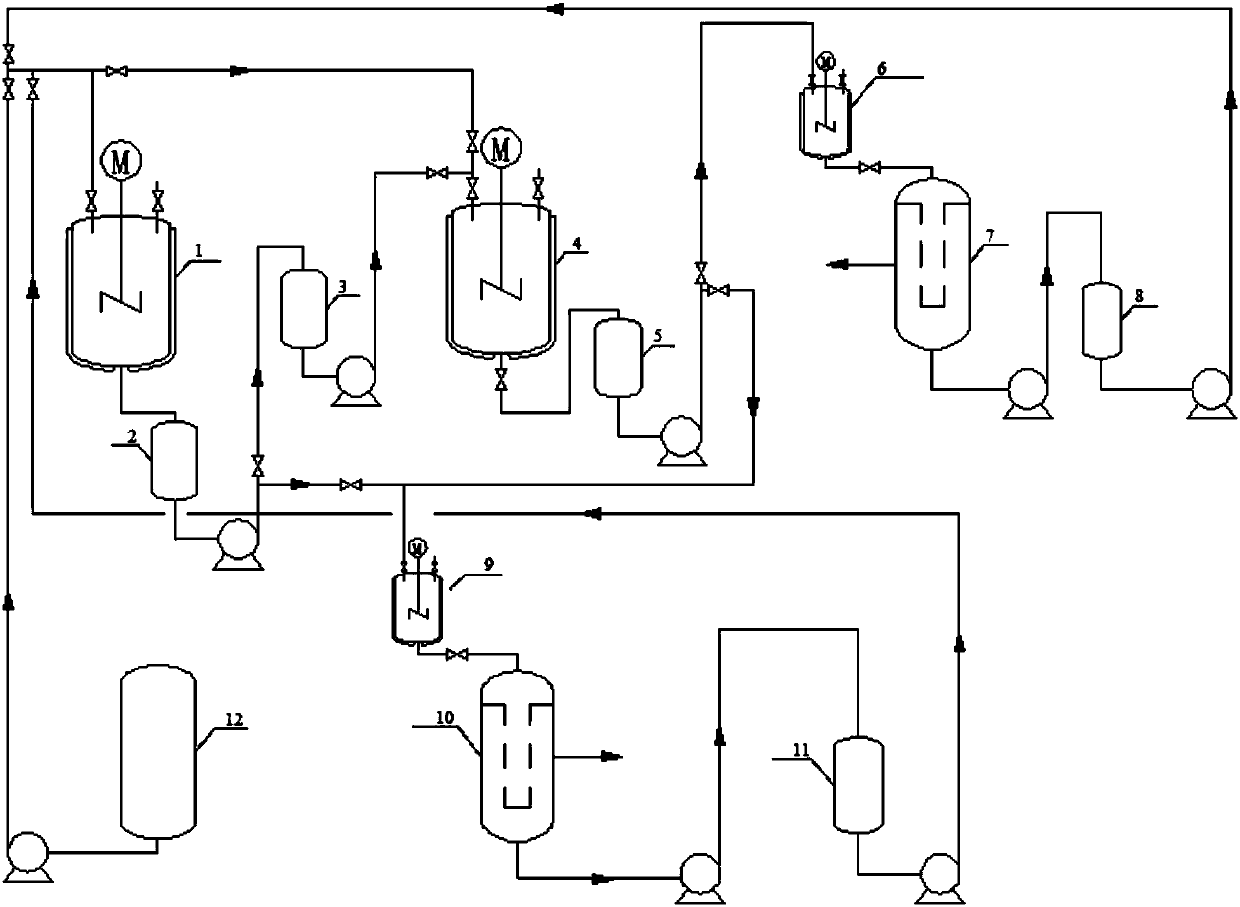
[0024] The experimental flow chart is illustrated in the patent drawings figure 1 : As shown in a 2-methyl-4-amino-5-(formylmethyl) pyrimidine pilot cycle hydrolysis flow chart, comprises the steps:
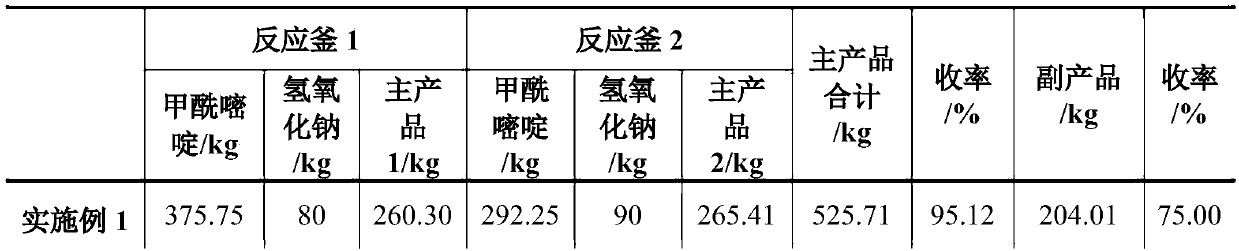
[0025] (1) Add 400L of distilled water in the reflux reactor 1, add 375.75kg of 2-methyl-4-amino-5-(formamidomethyl)pyrimidine and 80.00kg of sodium hydroxide from the solid feed port, Start stirring, control the temperature at about 110°C, and react for 2 hours. After the reaction, 2000 L of toluene was added to the reaction kettle 1 to extract the main product 2-methyl-4-amino-5-(aminomethyl)pyrimidine. After half an hour, the stirring was stopped, the layers were separated, and the organic phase and the aqueous phase were released.
[0026] (2) The water phase is put into the water phase storage tank 3 through the intermediate tank 2; the toluene phase is put into the cooling kettle 9 through the intermediate tank 2, cooled to about 0°C, and the product is separated out afte...
Embodiment 2
[0031] The 400L aqueous solution that is dissolved with excess sodium hydroxide left in the water phase storage tank 8 of Example 1 is transported in the reactor 1, and replaces 400L distilled water as a solvent for mechanical application. Other process conditions and operating procedures are as in Example 1, and the raw materials are added See Table 1 for the amount and product yield.
[0032] In the above-mentioned process example: the 2-methyl-4-amino-5-(aminomethyl)pyrimidine separated twice was combined to obtain 550.01kg, and the obtained product was detected by HPLC as 2-methyl-4-amino-5 The -(aminomethyl)pyrimidine content was 99.20%.
Embodiment 3
[0034] The 400L aqueous solution that is dissolved with excess sodium hydroxide left in the aqueous phase storage tank 8 of Example 2 is transported in the reactor 1, and replaces 400L distilled water as a solvent to apply mechanically. Other process conditions and operating procedures are as in Example 1, and the raw materials are added See Table 1 for the amount and product yield
[0035] In the above-mentioned process example: the 2-methyl-4-amino-5-(aminomethyl)pyrimidine separated twice was combined to obtain 551.32kg, and the obtained product was detected by HPLC as 2-methyl-4-amino-5 The -(aminomethyl)pyrimidine content was 99.30%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com