Specific modification of antibody by IgG-binding peptide
A residue and amino acid technology, applied in the field of production of the complex, can solve the problems of reaction yield impact, complex reaction steps, high development cost, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
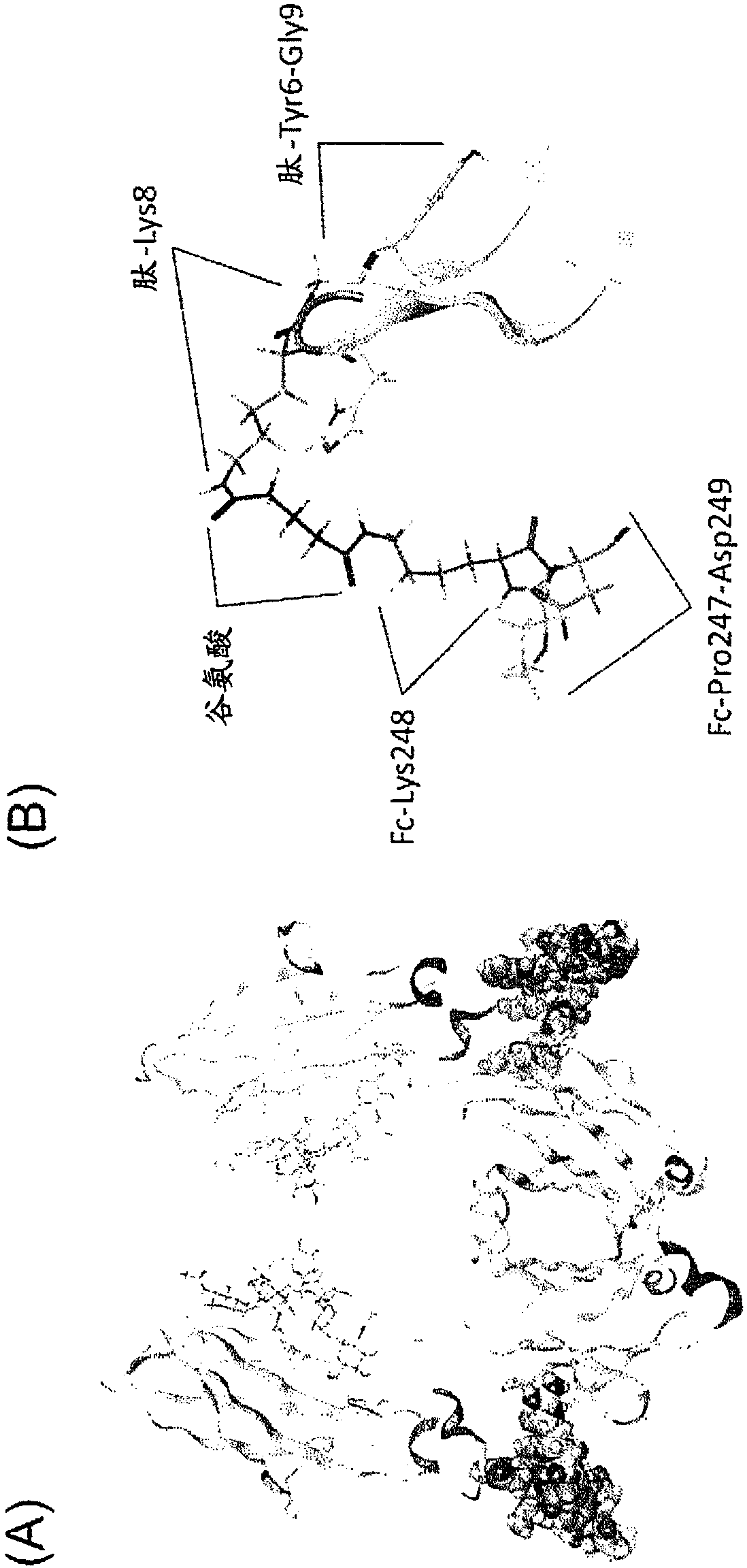
[0374] [Example 1: Analysis of X-ray crystal structure of a complex of IgG-binding peptide and IgG]
[0375]
[0376] (1) Preparation of IgG binding peptide solution
[0377] By using the peptide solid-phase synthesis method of the F-moc method, according to the conventional method to prepare the G(HC)DCAYHRGELVWCT(HC)H-NH 2 The sequence (SEQ ID NO:31, wherein, HC is homocysteine, the 4th and 14th two Cys, the 2nd and the 16th two homocysteine respectively form two Sulfur bond) cyclic homocysteine peptide. 0.8 mg of the prepared IgG-binding peptide powder was dissolved in 24 µL of 100% dimethylsulfoxide (Wako Pure Chemical Industries, Ltd.) to prepare an IgG-binding peptide solution.
[0378] (2) Preparation of a complex of Fc and IgG-binding peptide
[0379] In 20 mmol / L phosphate buffer (pH 7.0) containing 10 mM EDTA and 1 mM L-cysteine, the hinge of human IgG (Chugai Pharmaceutical) was digested with papain (manufactured by Roche) at 37 °C. )part. Next, the human...
Embodiment 2
[0390] [Example 2: Preparation and properties of peptides for labeling]
[0391]
[0392] Use biotin (Biotin) or 5 / 6-Tamura succinimidyl ester (TAMURA succinimidyl ester) (AnaSpec company) (fluorochrome) to amino-PEG4 synthesis peptide GPDCAYHXGELVWCTFH (SEQ IDNO:2) (wherein, The C-terminus is amidated) was synthesized according to a conventional method using Fmoc solid-phase synthesis. After removal of the protecting group, intramolecular S-S bond formation was performed under oxidative conditions in aqueous solution at pH 8.5 using reverse phase HPLC at a flow rate of 1.0 ml / min through a gradient of 10% to 60% acetonitrile containing 0.1% TFA Elution purifies peptides with intramolecular S-S bonds.
[0393]100 μL of a DMF solution containing 1 mM of the purified IgG-binding peptide was mixed with 100 μL of an acetonitrile solution of 100 mM DSS or DSG (Thermo Fisher Scientific), and reacted overnight at room temperature. After diluting the reactant to 2.5 times with 0.1...
Embodiment 3
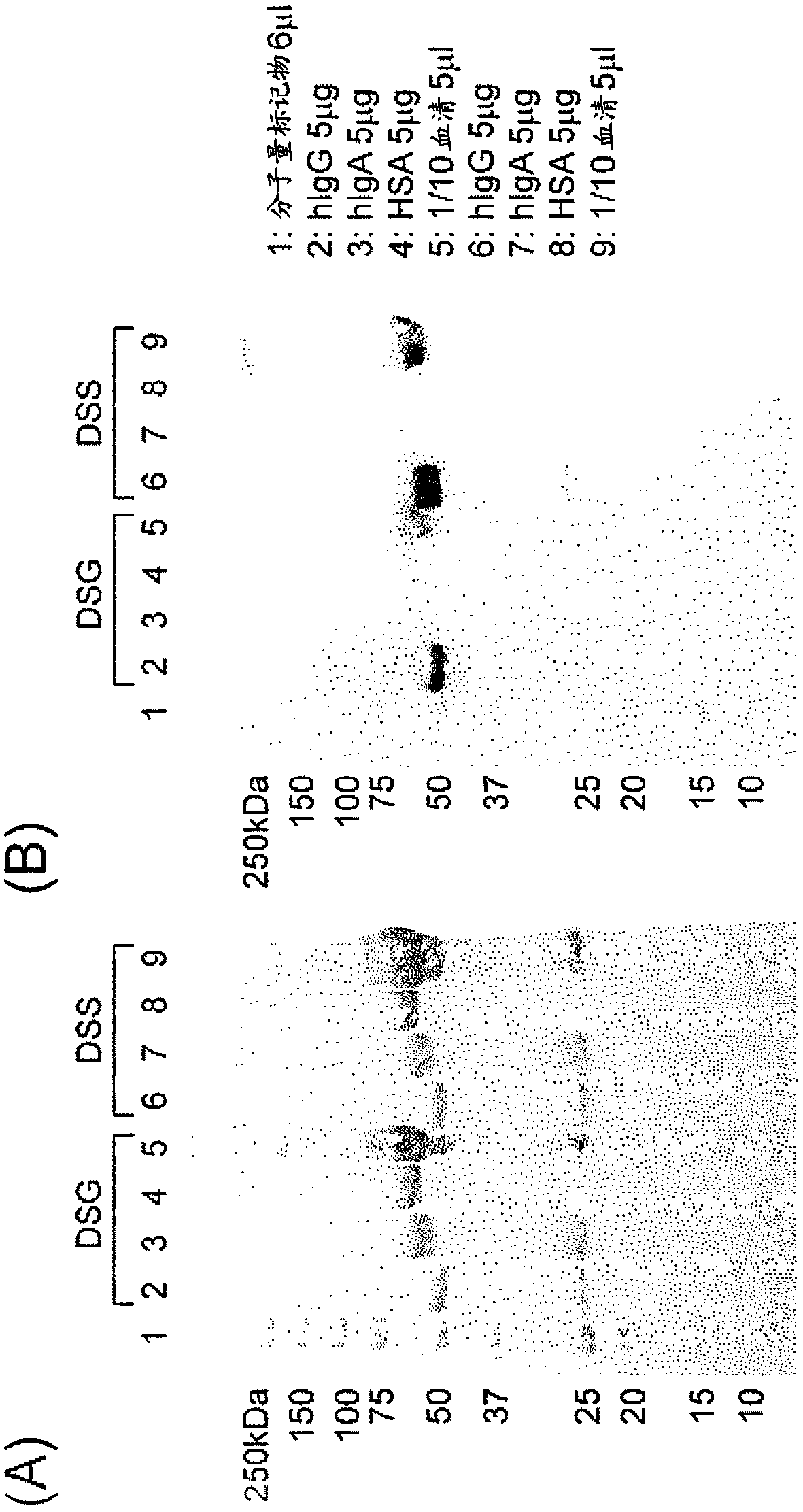
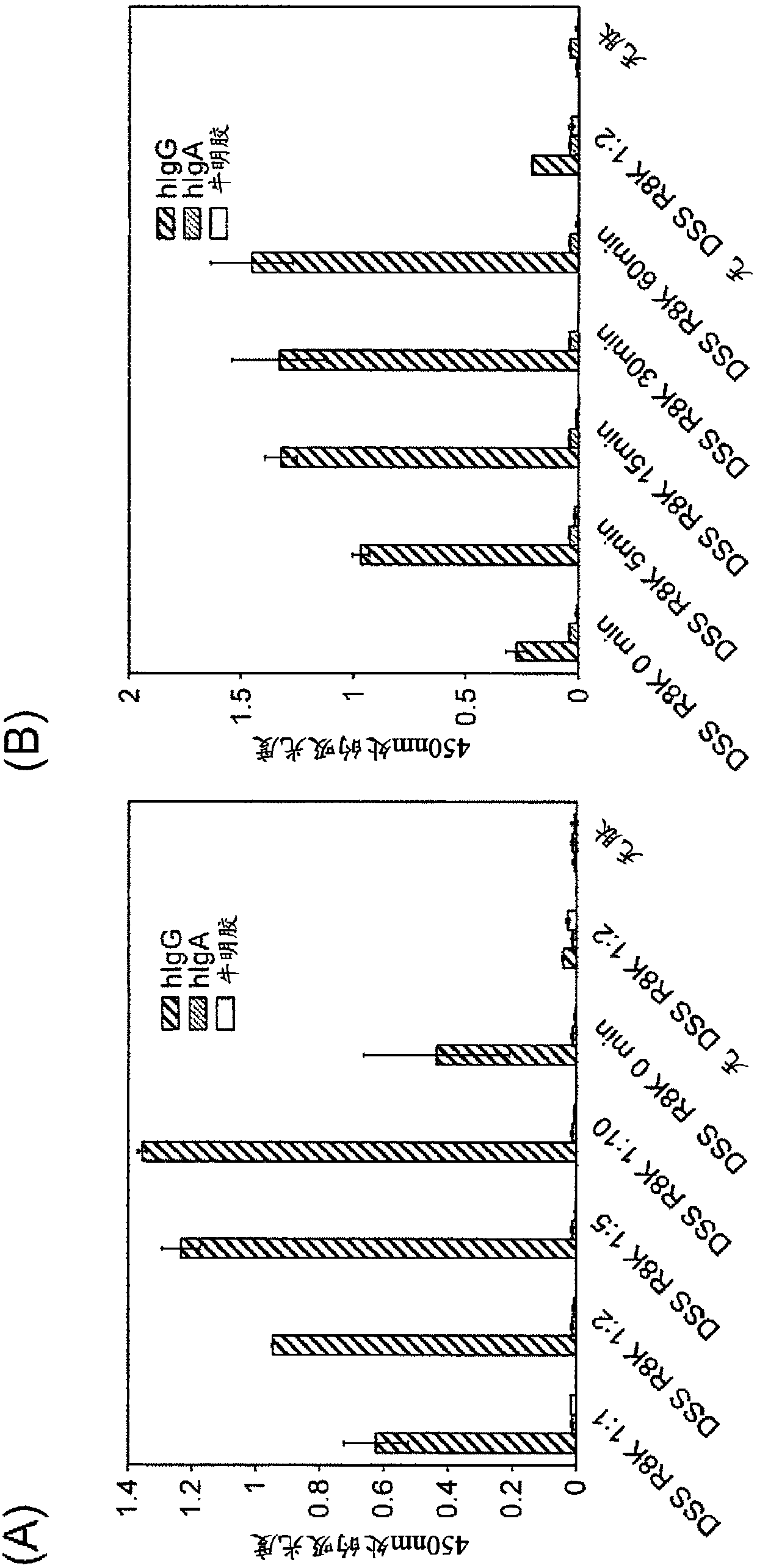
[0400] [Example 3: Specific modification of human IgG-Fc using IgG-binding peptide]
[0401]
[0402] A labeling reagent peptide modified with DSS or DSG to an IgG-binding peptide (type I (R8K)) with biotin-PEG4 added to the N-terminus was prepared by the same method as in Example 2, and reacted with human IgG Fc to study human Labeling reaction of IgG Fc. That is, after purifying the IgG-binding peptide (R8K) (200 pmol / 5 μL in 0.1% TFA) reacted with excess DSS or DSG using a reversed-phase column by the same method as in Example 2, acetonitrile was removed under reduced pressure, and then about 1 / 8 of 0.5M Na 2 HPO 4 For neutralization, immediately add protein samples (hIgG (Chuwai Pharmaceutical), hIgA (Athens Research & Technology), HSA (Sigma-Aldrich), or serum (collected from healthy people)) at a molar ratio of 10 times (each 40pmol / 5μL, Serum was diluted 10-fold with PBS), and after making the final volume to 20 μL with PBS, it was left at room temperature for 5 mi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com