Preparation method of tedizolid phosphate composition tablets
A technology of tedizolid phosphate and composition, which is applied in the field of preparation of tedizolid phosphate composition tablets, can solve the problems that the stability of drug crystal form greatly affects the curative effect and antibacterial effect, etc., so as to improve fluidity and Effects of compressibility, small difference in loading capacity, and improved antibacterial activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0029] The preparation method of tedizolid phosphate tablet:
[0030] (1) Raw material preparation: tedizolid phosphate, pregelatinized starch, sucrose, tyrosine, polacrilin potassium, mixed and pulverized, repeatedly passed through a 100 mesh sieve twice; povidone K30 was prepared into a 5% solution , as an adhesive;
[0031] (2) Granulation: Add the pulverized and sieved material into a wet granulator and mix evenly, the mixing speed is 400-600 rpm, the mixing time is 5-6 minutes, then add 5% povidone K30 solution For making soft materials, granulate with 18 mesh sieve, dry at 50°C for 40 minutes, and granulate with 20 mesh sieve;
[0032] (3) Total mixing: place the sized granules and sodium lauryl sulfate in a three-dimensional mixer and mix evenly. The mixing speed is 10-15 rpm, and the mixing time is 8-10 minutes.
[0033] (4) Tablets.
experiment example 1
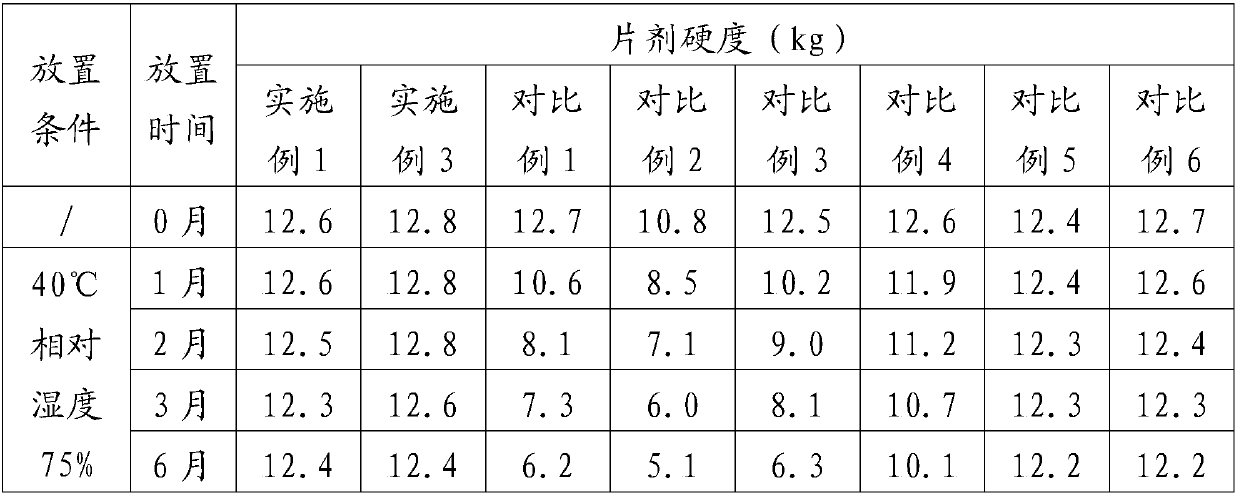
[0066] The investigation of experimental example 1 tablet hardness stability
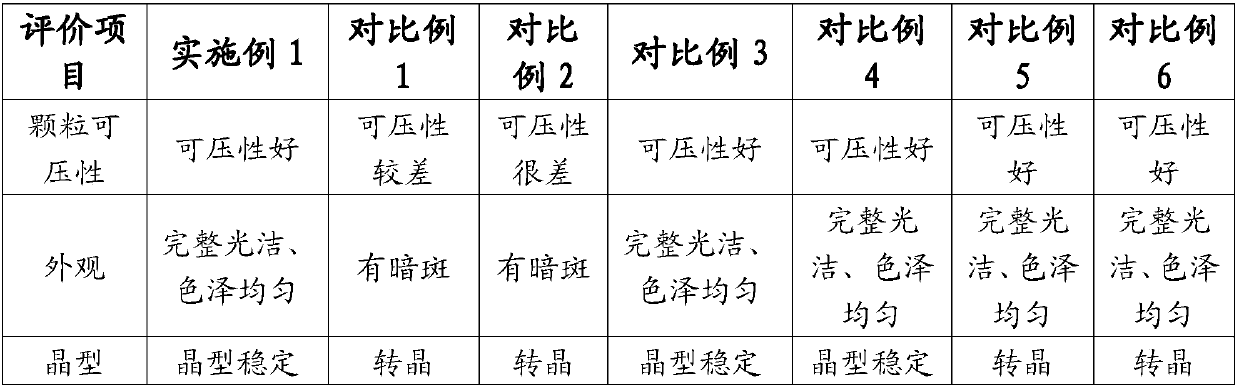
[0067] Get the prepared tablet samples of Example 1, Example 3, Comparative Example 1, Comparative Example 2, Comparative Example 3, and Comparative Example 4, under packaging (aluminum-plastic packaging, put in a carton), at 40 ° C, Place it under 75% relative humidity for 6 months, take samples at 0, 1, 2, 3, and 6 months, and measure the tablet hardness. The results are shown in Table 1.
[0068] Table 1 tablet hardness investigation result
[0069]
[0070] The accelerated test was conducted for 6 months, and the hardness measurement results showed that the hardness of the tablet of the present invention remained unchanged under long-term storage conditions, and the stability of the preparation was significantly improved.
experiment example 2
[0071] Experimental Example 2: Efficacy and Safety Evaluation of Treatment for MRSA Pneumonia
[0072] Refer to the patent CN105085570A method for detection, and the test results are shown in Table 2-4.
[0073] Table 2 Comparison of Clinical Curative Effects of Patients (Example)
[0074] group
[0075] Table 3 Comparison of Bacteriological Curative Effects of Patients (Example)
[0076] group
[0077] Table 4 Comparison of adverse reactions in two groups of patients (example)
[0078] group
[0079] The comparison difference of embodiment and comparative example all has statistical significance (P<0.05), can find out that the curative effect of tedizolid phosphate composition of the present invention and untoward reaction are all better than prior art, significantly to MRSA antibacterial activity improve.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com