Slow-release cutaneous penetration patch used for vulva seborrheic dermatitis
A technology for vulvar fat and dermatitis, applied in skin diseases, drug combinations, pharmaceutical formulations, etc., can solve the problems of ulcer surface enlargement, toxic side effects, deepening and other problems, and achieve the effect of good curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
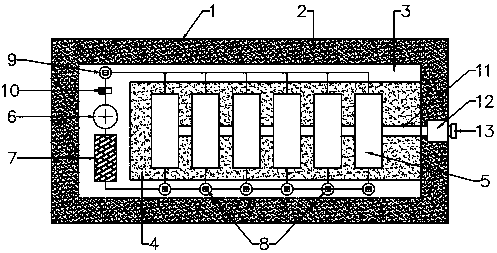
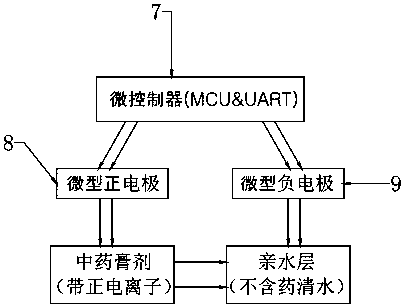
Image
Examples
Embodiment 1
[0072] The raw material composition and the weight of active ingredient of medicine of the present invention are:
[0073] 20-30 parts of amaranthus, 20-30 parts of gymnasium vine, 20-30 parts of small tree leaves, 15-30 parts of sedge,
[0074] 15 to 30 parts of scorpion vine, 10 to 30 parts of Dongfeng grass, 10 to 25 parts of Kochia scoparia, 10 to 25 parts of white moss bark,
[0075] 10-20 parts of suet, 10-20 parts of snake grape root, 9-15 parts of licorice, 9-15 parts of sea pine nuts,
[0076] 6-15 parts of Asparagus, 6-15 parts of Sophora japonica, 6-12 parts of Witch Root, 6-12 parts of Red Heliotrope,
[0077] 6-12 parts of wild pepper leaves, 4-10 parts of Fructus Lily, 4-10 parts of linseed, 3-9 parts of cnidium,
[0078] 3-9 parts of Jade Butterfly, 3-6 parts of Osmanthus fern, 3-6 parts of Ling Xiaohua, and 1-3 parts of borneol.
[0079] The processing steps of the pharmaceutical preparation method of the present invention are:
[0080] (1) Combine amaranth...
Embodiment 2
[0094] The raw material composition and the weight of active ingredient of medicine of the present invention are:
[0095] 25-30 parts of amaranthus, 25-30 parts of gymnasium vine, 20-26 parts of small tree leaves, 18-25 parts of sedge,
[0096] 18 to 25 parts of scorpion vine, 15 to 25 parts of Dongfeng grass, 12 to 20 parts of Kochia scoparia, 12 to 20 parts of white moss bark,
[0097] 12-18 parts of suet, 12-18 parts of snake grape root, 12-15 parts of licorice, 10-13 parts of sea pine nuts,
[0098] 8-13 parts of Asparagus, 8-12 parts of Sophora japonica, 8-10 parts of Heliconia root, 8-10 parts of Red Heliotrope,
[0099] 6-10 parts of wild pepper leaves, 6-9 parts of Sapphire, 5-8 parts of linseed, 5-8 parts of cnidium,
[0100] 5-7 parts of Jade Butterfly, 4-6 parts of Osmanthus fern, 3-5 parts of Ling Xiaohua, and 1-2 parts of borneol.
[0101] The process steps, usage and dosage of the medicine preparation method in this embodiment are the same as those in Example...
Embodiment 3
[0103] The raw material composition and the weight of active ingredient of medicine of the present invention are:
[0104] 28 parts of Amaranth, 28 parts of gymnasium vine, 24 parts of small tree leaves, 22 parts of sedge,
[0105] 22 parts of scorpion vine, 20 parts of Dongfeng grass, 18 parts of Kochia scoparia, 16 parts of white moss bark,
[0106] 16 parts of suet, 15 parts of snake grape root, 13 parts of licorice, 12 parts of sea pine nuts,
[0107] 10 parts of Asparagus, 10 parts of Sophora japonica, 9 parts of Wingwort root, 9 parts of Red Tiankui,
[0108] 8 parts of wild pepper leaves, 7 parts of Sapphire, 7 parts of linseed, 6 parts of cnidium,
[0109] 6 parts of Jade Butterfly, 5 parts of Lemongrass Fern, 4 parts of Lingxiaohua, and 1 part of Borneol.
[0110] The process steps, usage and dosage of the medicine preparation method in this embodiment are the same as those in Example 1.
[0111] clinical information:
[0112] 1. Case selection
[0113] 80 patie...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com