Bivalent hepatitis B vaccine and preparation method thereof
A technology of hepatitis B vaccine and bivalent hepatitis B, which is applied in the field of bivalent hepatitis B vaccine and its preparation, can solve the problems of immune evasion and achieve the effect of preventing infection and good protection effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
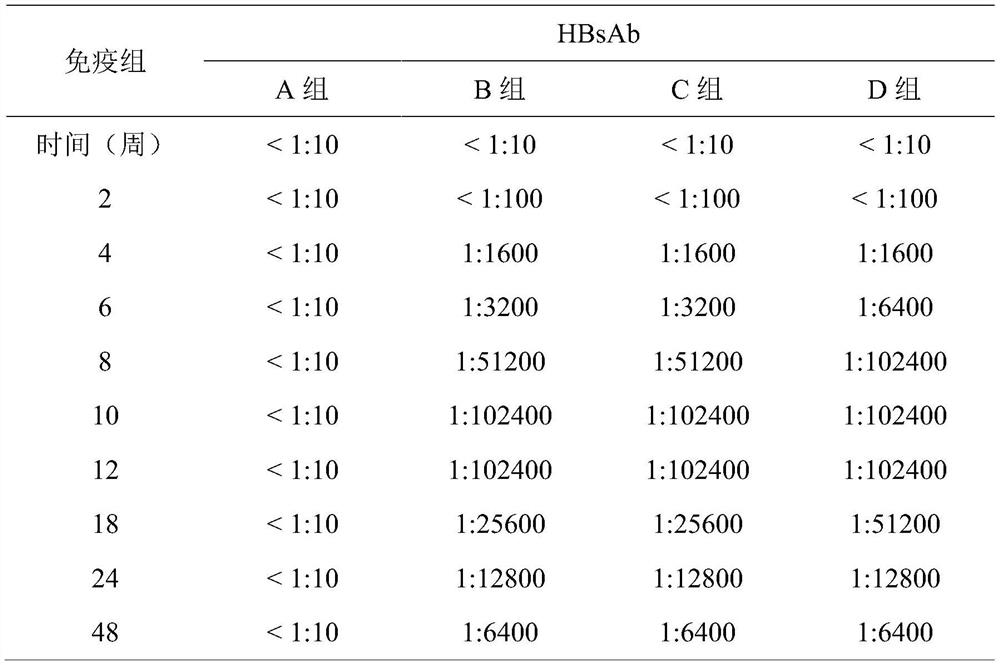
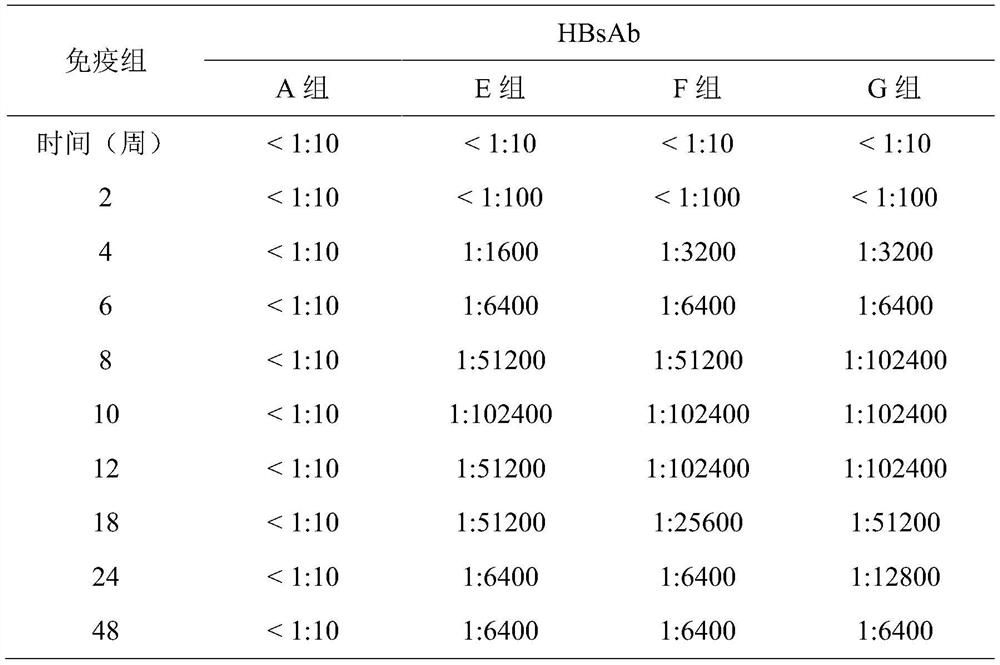
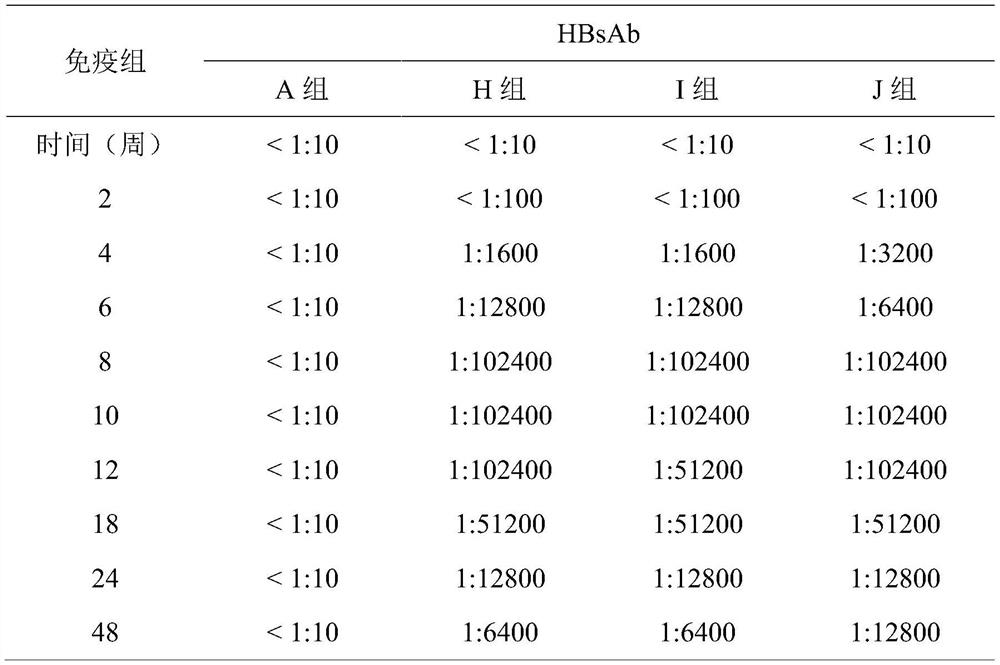
[0027] A bivalent hepatitis B vaccine, based on its total volume, comprising recombinant wild-type hepatitis B virus surface antigen (recombinant w-HBsAg, concentration of 10 μg / ml), recombinant mutant type hepatitis B virus surface antigen (recombination m-HBsAg, concentration of 10μg / ml) and aluminum hydroxide gel (0.6mg / mL concentration). Recombinant w-HBsAg or recombinant m-HBsAg is a genetically engineered recombinant protein expressed by yeast cells, mammalian cells or other available cells. Wherein, the recombinant w-HBsAg contains 226 amino acid sequences of HBsAg of wild-type HBV, and its amino acid sequence is shown in SEQ ID No.1. The recombinant m-HBsAg contains 226 amino acid sequences of mutant HBsAg at three mutation sites of L21S, I126S and G145R, and its amino acid sequence is shown in SEQ ID No.2.
[0028] Its preparation method is as follows:
[0029] (1) Mix the recombinant w-HBsAg and the recombinant m-HBsAg with the aluminum hydroxide gel solution respe...
Embodiment 2
[0032] A bivalent hepatitis B vaccine, based on its total volume, comprising recombinant wild-type hepatitis B virus surface antigen (recombinant w-HBsAg, concentration of 20 μg / ml), recombinant mutant type hepatitis B virus surface antigen (recombinant m-HBsAg, concentration of 20μg / ml) and aluminum hydroxide gel (1.0mg / mL concentration). Recombinant w-HBsAg or recombinant m-HBsAg is a genetically engineered recombinant protein expressed by yeast cells, mammalian cells or other available cells. Wherein, the recombinant w-HBsAg contains 226 amino acid sequences of HBsAg of wild-type HBV, and its amino acid sequence is shown in SEQ ID No.1. The recombinant m-HBsAg contains 226 amino acid sequences of mutant HBsAg at three mutation sites of L21S, I126S and G145R, and its amino acid sequence is shown in SEQ ID No.2.
[0033] Its preparation method is carried out with reference to embodiment 1.
Embodiment 3
[0035]A bivalent hepatitis B vaccine, based on its total volume, comprising recombinant wild-type hepatitis B virus surface antigen (recombinant w-HBsAg, concentration of 60 μg / ml), recombinant mutant type hepatitis B virus surface antigen (recombinant m-HBsAg, concentration of 60 μg / ml), aluminum hydroxide gel (1.5 mg / mL concentration), and 0.5-0.7 w / v% gelatin. Recombinant w-HBsAg or recombinant m-HBsAg is a genetically engineered recombinant protein expressed by yeast cells, mammalian cells or other available cells. Wherein, the recombinant w-HBsAg contains 226 amino acid sequences of HBsAg of wild-type HBV, and its amino acid sequence is shown in SEQ ID No.1. The recombinant m-HBsAg contains 226 amino acid sequences of mutant HBsAg at three mutation sites of L21S, I126S and G145R, and its amino acid sequence is shown in SEQ ID No.2.
[0036] Its preparation method is as follows:
[0037] (1) Mix the recombinant w-HBsAg and the recombinant m-HBsAg with the aluminum hydrox...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com